
Microsystems for electromechanical stimulations to engineered cardiac tissues
Introduction
Cardiovascular diseases are the leading cause of death globally with an estimated 8 million deaths per year. These diseases cause severe and irreversible damage to the tissues, valves and vasculature that comprise the heart. The lack of donors and risk of immune rejection limits the sufficiency of heart transplantation as a treatment option. As an alternative, stem cell therapy is being intensively studied for regenerative medicine, disease modeling and drug discovery. The effectiveness of cardiac cell therapy is highly dependent on the maturity and viability of CMs, as well as the ability to electromechanically integrate with the native CMs. Electromechanical integration of CMs is necessary to drive basic physiological functions such as intracellular Ca2+ handling, contractile force generation and relaxation (1,2). The use of human induced-pluripotent stem cells (hiPSCs) has been proposed for the development of autologous cell therapy, but generation of high-quality transplantable hiPSC-CMs has not been thoroughly investigated. While hiPSC-CMs show great potential, they are also phenotypically immature and have different features from healthy adult-CMs. As a result, introducing immature hiPSC-CMs into damaged cardiac tissue as a means of tissue repairing and regeneration raises the risks of arrhythmogenicity (3-5), because (I) nascent hiPSC-CMs do not have the natural automaticity (pacing) of their mature counterparts; (II) they have sub-optimal contractile capability; and (III) they have insufficient intercellular electrical propagation due to inadequate structural alignment and Cx43 expression for functional integration of hiPSC-CMs with the myocardium.
Researchers have attempted to engineer in vitro cardiac model systems using hiPSC-CMs. The most basic models consist of 2D monolayers of hiPSC-CMs cultured under static conditions. Due to the simplicity, these 2D models are suffering from little structural similarity to the normal 3D heart tissues. Additionally, the static culture conditions cannot mimic the physiological mechanical stress, which plays an essential role in the determination of the phenotypic morphology and cell alignment. These factors are therefore critical to be considered, when designing in vitro cardiac tissue models that are physiologically and clinically applicable. For example, drug cardiotoxicity screening requires high efficiency and human relevance. Therefore, researchers are developing in vitro microphysiological systems (MPS) that can create physiologically functioning cardiac tissues, which potentially provide a solution to the various challenges in small scale 2D cultures as well as an in vitro alternative to whole animal modeling (6). The technological aim of MPS is to mimic the native tissue environment by replicating the fundamental organ-specific conditions in stimulatory cell/tissue culture devices (7). These devices are inoculated with primary cells, or tissue specific cells derived from hESCs or hiPSCs (8,9) and stimulated to recapitulate native cellular behaviors. The miniaturization of these tissue systems offers several advantages, such as superior control over the local cellular microenvironment, reduction in the use of compounds, reagents and cells in a cost-effective manner (10-12). MPS can also be personalized to an individual for precision medical diagnoses and treatment.
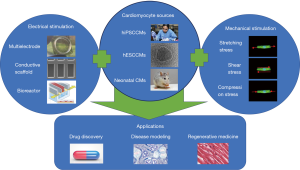
MPS are commonly single, perfused microfluidic chambers containing a single or multiple cell types. They are used to recapitulate the structure, function, microenvironment and heterogeneity of cells of one particular tissue. These systems are also flexible, allowing incorporation of physical stimuli, such as cyclic strain, fluid shear stress, electrical stimulations and mechanical compression experienced by the organ of interest. To recapitulate the in vivo physiological conditions, current cardiac MPS have been created by combining the appropriate materials, suitable topographical and structural cues, continuous perfusion of media, and long-term electromechanical stimulation (13-15). These cardiac MPS can also integrate various materials with different properties, such as elasticity, stiffness, surface topography, and electrical conductivity to optimize extracellular microenvironments (16-20). In this review, we will discuss how both mechanical and electrical stimulation can be applied to the CMs in a microsystem and whether the stimulation can induce physiological maturation of hiPSC-CMs to mimic adult cardiac tissues (Figure 1). For mechanical stimulation, we will focus on the influence of mechanical stretch, shear stress and compressional force on the CMs. For electrical stimulation, we will focus on the technologies of bioreactor, MEA, and conductive scaffolds for applying electrical stimulation to the cardiac constructs.
Heart physiology and hiPSC-CMs maturation
The heart is a muscular organ of the cardiovascular system and acts as a physiological pump that circulates blood throughout the body. It is comprised primarily of highly organized, elongated, hypertrophied CMs that are aligned and electrically coupled with each other. The contractile unit of CMs is the myofibril, which is composed of myofilaments that slide along each other to produce a muscle contraction. Muscular contraction is triggered from an electrical signal generated by the sinoatrial nodes in the atria. This signal generates an action potential (AP) in the downstream CMs, where the electrical signals are then propagated between neighboring cells through gap junctions. These impulses in the CMs is the result of the increase and decrease of the intracellular Ca2+, initiated by the depolarization of the sarcolemma and is sustained by the release and uptake of the Ca2+. The mechanical contraction is triggered by electrical depolarization through an intracellular calcium dependent process called “excitation-contraction coupling”. Cardiac muscle contraction is caused by the CM shortening to generate force along their long axis in the sarcomeres.
hiPSC-CMs offer distinct advantages for cardiac disease modeling. hiPSC-CMs, however lack key features of adult CMs, which limits their capability to model physiological responses resulted from adult cardiac diseases. For this reason, it has become essential to assess and promote the maturity of hiPSC-CMs. hiPSC-CMs are structurally similar to fetal CMs, which are smaller, round shape, and approximately 5–10 µm in diameter, while adult CMs are large and cylindrical (ventricular cells are approximately 150 µm × 10 µm) (21-25). In addition, adult CMs are multi-nucleated with extensive t-tubule networks, while hiPSC-CMs are mono-nucleated with no distinct t-tubule networks (26). A hallmark of mature mammalian ventricular myocardium is the positive exhibition of the Frank-Sterling mechanism and the force-frequency relationship (FFR), both of which are used to assess the contractile performance of a healthy myocardium (27,28). A positive FFR is dependent on the maturity of the intracellular calcium stores, the sarcoplasmic reticulum (SR) and T-tubulation, which are the indicators of rapid recycling of intracellular Ca2+ during systole and diastole (29-31). Post-rest potentiation (PRP) is also a physiological indicator of the developmental state of the CMs. The PRP is contributed by influx of transmembrane Ca2+, exchange of Na+/Ca2+ and release of intracellular storage of Ca2+. Mature CMs exhibit an increase of PRP due to the increase of release of intracellular Ca2+ from ER and reduced Na+/Ca2+ exchange activity (32,33). The excitation-contraction coupling of hiPSC-CMs tends to be slower, since the Ca2+ is released into the cells through the sarcolemma rather than from SR as in adult CMs (34-36). Eventually, adult CMs lose the ability to spontaneously beat without external electrical stimuli. These electrophysiological characteristics can be used to assess the hiPSC-CM maturity. The maturity of CMs can also be assessed by gene expression of myosin, titin, troponins and calcium handling proteins (37-39). Mature CMs have a different composition and organization of their cytoskeleton and contractile apparatuses. Additionally, mature CMs utilize fatty acid oxidation for metabolic activities, which produces higher oxidative energetic profile comparing to glycolytic oxidation utilized by hiPSC-CMs. Since adult CMs are highly metabolically active, mature CMs show a significant larger number of mitochondria in the cytoplasm, comparing to the hiPSC-CMs.
Postnatal maturation of the human heart is achieved over a period of 6 years, suggesting that an extended period is required to achieve completed maturation of CMs. In hiPSC-CM maturation, continued systemic morphological changes (multi-nucleation, increase in cell size, and sarcomere length) and functional parameters [slowed contraction kinetics, increase in calcium kinetics and action potential duration (APD)] were observed over a 4-month-long culture period (34). This indicated that extended period of culture time is required for the mechanical and structural rearrangements that appear necessary to build the CM architecture to achieve adult-like CM features. This extended time makes it difficult to engineer physiologically relevant adult cardiac tissue in an efficient and robust manner, making it necessary to develop methods to accelerate hiPSC-CM maturity.
External stimulation can be used to induce CM maturation, since in vivo CMs are constantly subjected to electrophysiological and mechanical stimuli (40). In addition, cardiac tissues are both electrically and mechanically active, exhibiting both electrophysiological and contractile properties. This suggests that supplementation of these stimulatory factors to immature CMs can potentially induce changes to tissue structure, functions and gene expression. Electrically stimulated ventricular neonatal rat CMs (nrCMs) exhibited increased APD, maximal Vmax, Na+/Ca2+ exchanger expression and overall mitochondrial content and activity. Other than electrical stimulation, the mechanical cues, such as stiffness and surface topography, also influence the CM maturation. Current research has shown that when nrCMs were cultured on polyacrylamide-based gels that provided stiffness, similar to the native matrices of the heart, the CMs exhibited greater mechanical force. Many other attempts on maturation of CM have reconstructed the 3D physiologically relevant tissue structure that improves the alignment, elongation and functions of the hiPSC-CMs with regulated expression of sarcomeric genes (TNNI3, MYH6 and MYH7) and ion channel genes (KCNH1, KCNH2 and RYR2). Therefore, in this review, we will focus on the utilization of mechanical and electrical stimulation to improve the hiPSC-CM functions and accelerate the maturation process.
Besides electromechanical environments surrounding the cardiac tissues, thick myocardial tissues require microvascular networks to diffuse oxygen and nutrients, remove waste products, and promote vessel anatomist, since thick solid tissues become highly vulnerable within hours without oxygen supply. Most micro-tissues composed of parenchymal cells are studied in the absence of vasculature. To combat the conflicting material requirements of permeability and stability, Zhang et al. developed the AngioChip, a biodegradable scaffold with built-in branching micro-channel networks to support perfusable vasculature in contracting tissues (41). AngioChip achieved tissue endothelialization within one day, prior to parenchymal seeding. To promote vascularization in vitro, Vollert et al. scaled up a miniaturized fibrin-based EHT to a larger six-well format with flexible silicon posts holding each EHT (42). The perfusion of oxygen, measured with a micro-sensor, rapidly increased from 10% to 12% within the microchannels. Bioprinting technology was leveraged to engineer endothelialization inside myocardial tissues by incurring endothelial migration towards microfibers, forming a layer of confluent endothelium (43). The novel bioprinting strategy demonstrated microfibrous networks emulating blood vessels can serve as a vascular bed for endothelialized tissues besides myocardium. Fibrin-based mini-EHTs were generated from a transgenic mouse line (Cdh5-CreERT2 × Rosa26-LacZ), in which ECs were specifically and inducible labeled by applying tamoxifen (ECiLacZ). ECiLacZ EHTs showed a dense X-gal-positive vessel-like network with distinct tubular structures, and developed spontaneous and regular contractility with forces up to 0.1 mN (44).
Mechanical stimulation to CMs
The heart is constantly subjected to mechanical stimulations that are induced by normal contraction of the heart muscle. Mechanical stimulations in vivo regulate mechanotransduction signaling pathways that direct cell morphogenesis and influence the tissue functions. Applying mechanical stimulation to cardiac tissues has been found to promote cell alignment, activate signaling pathways, and strengthen ECM components (45,46). Additionally, these signals lead to the production and release of growth factors, and other proteins that enhance myocardial repair (47,48). Mechanical stimulation can be applied by stretching, compression, or shear stress. This review will discuss various methods of mechanical stimulation integrated within the engineered platforms, which promote cardiac tissue development and functions (Table 1). To emphasize, mechanical stimulation is essential not only to engineering hiPSC-CMs, but also to maintaining primary adult cardiac tissues, which tend to lose their contractile phenotypes within 24–72 hours as a result of myofibril breakdown (55).
Table 1
| Systems | Stimulation mechanisms | Cell types | Key results/advantages | Ref. |
|---|---|---|---|---|
| 2D system of a silicone membrane sheet fixed on a Teflon bar that could be moved freely in the X direction | Cyclic uniaxial stretch | nrCMs | Increased CV over 6 hours of pulsatile stretch slight | Zhuang et al. (49) |
| Increase in the AP amplitude | ||||
| Increase in the Cx43 and N-cadherin expression at 6 hours | ||||
| Alignment of cells in the direction of stretch | ||||
| 3D PDMS construct on mounted motorized mechanical stage capable of applying horizontal transverse force | Cyclic uniaxial stretch | nrCMs | Increased contraction amplitude | Sidorov et al. (50) |
| Longitudinally aligned cardiomyocytes | ||||
| Well-developed sarcomeric structure | ||||
| Expected transmembrane action potentials | ||||
| 3D tissue constructs on rectangle loading posts mounted on a motorized baseplate | Cyclic stress conditions | hESC-derived cardiac progenitors | Increased passive and active force magnitude | Ruan et al. (51) |
| Increased passive stiffness | ||||
| Increased force production with increasing Ca2+ concentration | ||||
| 48-well plate bioreactor with silicone gas exchange membranes horizontal compression platform operated by crankshaft engine | Compression pressure and perfusion-mediated fluidic shear stress | nrCMs | Elongated cells with striated, organized myofibrils and well-defined Z-lines | Shachar et al. (52) |
| Elevated secretion of bFGF and TGF-beta, cardiac muscle proteins, gap-junction proteins | ||||
| 2D system; a pump, collapsible pulsatile valve and an adjustable hemostatic valve integrated into thin and flexible silicone membrane | Passive stretch; pulsatile flow | embryonic cardiomyoblasts | Higher proliferation rates, better alignment, higher contractility, and beat rate | Giridharan et al. (53) |
| 3D tissue constructs, an array of hanging posts to confine cell-laden gels, a pneumatic actuation system to induce homogeneous uniaxial cyclic strains | Cyclic uniaxial strain | nrCMs and hiPSC-CMs | Early-synchronized beating and better contractile capability | Marsano et al. (54) |
| increase of junction complexes between cardiac cells | ||||
| Complex sarcomere structures | ||||
| Lower ET and higher MCR | ||||
| Increased amplitude of contractility |
PDMS, polydimethylsiloxane; nrCMs, neonatal rat cardiomyocytes; hESC, human embryonic stem cell; hiPSC-CMs, human induced pluripotent stem cell derived cardiomyocytes; MCR, maximum capture rate; ET, electrical threshold; CV, conduction velocity.
Contractile force measurement
The contractile force of CMs is an important function of the heart to ensure that blood can be pumped throughout the body. Measuring the contractile forces is, therefore, an important functional assessment of the CMs. The contractile force of CMs can be measured in various ways, such as by optical mapping techniques, atomic force microscopy and traction force spectroscopy (56). Beussman et al. used microposts that consisted of vertical cantilever beams made of silicone to measure the contractile force of the cells (57). These microposts were functionalized with ECM proteins, which allow the attachment and spreading of the CMs on the top of the micropost arrays. As the cells contract, the generated forces cause the microposts to deflect, and the deflection can be measured using live cell imaging and computational analysis. As such, these microposts can be used to quantify multi-directional forces from the CMs. The contractile force sensors were also developed based on flexible, silicone-based posts, which can report the forces based on the deflection of the posts recorded in real time with high-speed camera as demonstrated by Turnbull et al. (58). The twitch force could be calculated via the measurement of post defection based on the beam theory (Figure 2A). Schaaf et al. also showed that contraction force, relaxation force and frequency could be measured using slit formed agarose molds with two elastic silicon posts (60).
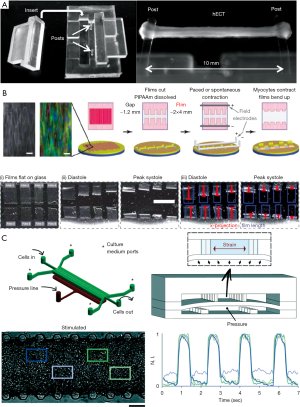
To measure and manipulate the mechanics of cardiac microtissues, Boudou et al. designed a microfabricated platform with two PDMS cantilevers embedded with fluorescent microbeads (61). nrCMs mixed with collagen I and fibrinogen was loaded into the platform and anchored to the top of the PDMS cantilevers. Linear bending theory and experimental measurements were used to report the load-displacement relationship to link the measured cantilever deflections to the amount of force generated by cardiac microtissues. By day 5, the beating cardiac microtissues tethered to the flexible cantilevers generated 2.39±0.24 mN of force, whereas cardiac microtissues tethered to the rigid cantilevers generated more force. The static tension was lower in flexible cantilevers than cardiac microtissues between rigid cantilevers (6.03±0.36 vs. 8.25±0.61 mN, respectively). In addition, they examined the influence of the bulk modulus of the collagen/fibrin matrix on the contractility of cardiac microtissues and observed that the static tensions were proportionally related to the collagen density. Furthermore, these PDMS cantilever-based devices were used to study titin-mutated cardiac microtissues for modeling dilated cardiomyopathy, a genetic disorder caused by mutations that truncate the sarcomere protein titin (62).
To establish a heart-on-chip system for cardiac contractile analysis, Grosberg et al. fabricated a chip from a protective film coated with a layer of PIPAAm on the surface (59). The nrCMs self-organized with respect to the fibronectin pattern, and formed an anisotropic monolayer. The films bend in response to systolic and diastolic stress from the contracting cardiac tissues (Figure 2B). The curvature of the film can be measured to compute the stress by considering the film as a two-layer plane strain beam. The volume growth method was used with the PDMS film and CM monolayer as the passive and active layers, respectively. The measurements of systolic and diastolic stresses were conducted under 37 °C and 2 Hz pacing. Although, the chips consisted of a 2D monolayer of CMs, which cannot fully replicate the 3D myocardium structure, the average systolic stress (20.7±5.6 kPa) and diastolic stress (8.0±2.0 kPa) lie within the stress range previously measured in isolated muscle strips (11–30 and 7–14 kPa, respectively). Using this “heart-on-chip” system, Wang et al. were able to model the mitochondrial cardiomyopathy, Barth Syndrome, in a controlled in vitro setting by using hiPSC disease lines derived from two individuals with this disease (63). The system could quantitatively measure the contractility of engineered myocardial tissues assembled from diseased or control hiPSC-CMs.
Stretching stress
Mechanical stretching has been used as a stimulus to enhance the organization, functionality, and strength of engineered tissues (64-66). Stretching allows control of cell morphology, proliferation, lineage commitment, and differentiation (67). The cells or tissues response to mechanical stretch may vary by cell types, loading modes, ECM properties and presence of soluble factors. However, cells and tissues have been extensively shown to respond to the external tensile forces and regulate internal cytoskeletal organization via mechanotransduction pathways that converts the mechanical signals into intracellular biochemical activities (68,69).
An in vitro study by Zhuang et al. demonstrated that 10% cyclic uniaxial stretch not only produced an upregulation of connexin-43 and N-cadherin at the intercellular junctions but also increased the electrical propagation velocity (49). The apparatus was a custom designed and fabricated device that applied linear pulsatile stresses on the cultured nrCMs. A silicone membrane sheet was fixed on the lateral borders to a Teflon bar that could be moved freely in the X direction along two stainless steel axes. nrCMs were seeded on a collagen coated silicone membrane and then subjected to pulsatile linear stretching for 1, 3 and 6 hours. The CV increased significantly from 27 cm/s for controls to 37 cm/s over 6 hours of pulsatile stretch with a slight increase in the AP amplitude. Additionally, there was a 3-fold increase in the Cx43 expression at 6 hours. The expression of N-cadherin was also increased in the cells under pulsatile stretch in a time course similar to the Cx43, when the cells were aligned along the direction of the stretch. These effects in gene expression and alignment remained unchanged even after prolonged periods of stretch relaxation.
The I-Wire engineered heart tissues (EHTs) were developed by Sidorov et al. as a cost-effective model capable of supplying sustained electrical, mechanical and chemical stimulations (50,70). A PDMS construct is casted to fit into a 6-well plate with thin ridges to create channels for the placement of two anchoring titanium wires. The EHTs were mounted on a motorized microscope stage capable of applying a transverse force horizontal to the mid-section of the anchored EHTs. The contractility of the EHTs was measured as a function of applied transverse force. Gradual increase of the transverse force up to 0.69 mN resulted in the stretching of the EHTs and led to the increase of contraction amplitude. The advantage of the I-Wire system is the ability to control the force applied to the EHTs, and the ability to measure the response to different transverse forces of by inserted cantilever probes. The contraction force amplitude decreased with increasing electrical pacing rate from 0.5–4 Hz, indicating a “negative staircase” behavior in force-frequency relationship, which might be resulted from the regulation of intracellular Ca2+.
The importance of mechanical stretch on the maturation of stem cell-derived cardiac tissue constructs was demonstrated by Ruan et al. (51). 3D tissue constructs created from human cardiac progenitors in collagen I gel mixed with basement membrane proteins. The constructs were subjected to no stress, static stress, and cyclic stress (1 Hz and 5% elongation) conditions through rectangle loading posts on a baseplate connected to a FX-4000T system (Flexcell). After two weeks, the tissue constructs under cyclic stress showed an increase of both passive and active force magnitude. The passive stiffness of the tissue constructs also increased from 0.22 kPa (no stress) to 0.47 kPa (static stress), and further to 0.71 kPa (cyclic stress). The engineered cardiac constructs had characteristics similar to the native myocardium, showing an increase of force production with increasing Ca2+ concentration.
Increasing substrate stiffness of 2D CM cultures has been conceptually connected with stretching of 3D EHTs, since they both apply a mechanical load to enhance CM contractile forces and promote overall maturation of the cells. Tzatzalos et al. (71) and Liaw et al. (72) have reviewed the effects of different stretching conditions, such as isometric, isotonic or auxotonic loads, on the structure and functions of EHTs. It has shown that auxotonic loading is superior, comparing to the other loading regimes, for increasing contractile forces and inducing CM maturation. Furthermore, dynamic culture conditions have been shown to increase the functional outputs of the EHTs (73).
Shear and compression stress
Hemodynamic cues are supplied from blood circulation in vivo and induce changes in strain and stress experienced by the tissues. These cues include stretching, shear and applied pressure by the constant fluidic pulsations. Thus, understanding and recapitulating these mechanical cues is critical factor for in vitro cardiac tissue modeling. Shachar et al. implemented both compression and perfusion-mediated fluidic shear stress to promote cardiac regeneration with organized myofibrils and striation (52). The rationale of this system is inspired by the mechanics of cardiopulmonary resuscitation. Compression and fluid shear stress on cardiac tissues was subjected via a custom-made bioreactor consisting of a 48-well plate with silicone gas exchange membranes located on its axial faces. The horizontal compression platform was operated using a crankshaft engine to apply up to 15% strain at the rate of 60 pulsations per minute. Piston movement and compression of the cells created a fluid dynamic in the system with compressive pressure and shear stress. The CMs subjected to continuous compression had round-shaped morphology with unorganized myofibrils and undefined Z-lines. Alternatively, intermittent compression resulted in elongated cells with striated, organized myofibrils and well-defined Z-lines. Secretion of bFGF and TGF-beta, cardiac muscle proteins, gap-junction proteins were elevated compared to the static conditions, which indicated the enhancement of cell-to-cell communication networks. Recurrent compression of the constructs over 4 days led to cardiac tissue regeneration with organized myofibrils resembling adult cardiac muscle tissues. These studies further strengthen the importance of medium perfusion due to compression and ability for simultaneous mechanical stimulation for high throughput assessment. One disadvantage of these mechanical cues is that the signal outcomes could not be distinguished from one another, as the fluid pattern is a direct effect from the piston movement in the well.
A cardiac cell culture system developed by Giridharan et al. consisted of a small cell culture chamber on a thin and flexible silicone membrane (53). A pump, collapsible pulsatile valve and an adjustable hemostatic valve were integrated to the thin membrane to simulate various loading conditions that the heart experiences early in development. CMs were seeded into the chamber, cultured for 4 days under standard static conditions, and then subjected to 8–15% passive stretch, approximately 10 mmHg peak pressure at a frequency of 2 Hz with the flow rate of 44 µL per cycle. The CMs under these stimulatory conditions exhibited higher proliferation rates, better alignment, higher contractility, and beat rate. This study demonstrated the need for mechanical stimulation in CM culture to maintain cell proliferation and enhance phenotypic characteristics.
Marsano et al. developed a heart-on-chip device to exert uniform cyclic strains to the micro-engineered cardiac tissues derived from both nrCMs and hiPSC-CMs (54). The device included an array of hanging posts to confine cell-laden gels, and a pneumatic actuation system to induce homogeneous uniaxial cyclic strains to the 3D tissue constructs (Figure 2C). Mechanical stimulation via cyclic straining promoted early-synchronized beating and better contractile capability in response to the electric pacing, because of the increase of junction complexes between cardiac cells. Cells exposed to cyclic strain showed more complex sarcomere structures compared to statically embedded CMs. Under electrical stimulation, lower ET and higher MCR were observed from the cardiac tissues that had been mechanically stimulated. Furthermore, the amplitude of contractility increased under cyclic uniaxial mechanical strain.
Electrical stimulation to CMs
Evidence suggests that electrical stimulation can regulate cardiac tissue maturation and subsequent enhancement of electromechanical contractile functions. Moreover, across various studies, in vitro electrical stimulation has been found to promote cardio-mimetic contractile synchronicity, enhance cellular proliferation, preferentially align CMs in the direction of the applied electric field, increase Cx43 expression, reduce ET and increase contraction amplitude. Therefore, electrical stimulation to the engineered cardiac tissues is being widely studied as a means of improving the functions and accelerating the maturation of hiPSC-CMs to advance their utility in cardiac tissue engineering (74). In this section, we will review works that have directly supplied electrical stimulation to the cardiac constructs via three major systems: MEA, conductive scaffolds, and bioreactors/microsystems (Table 2). In the following, we will discuss various approaches that have integrated electrical conduction components to enhance CM maturation.
Table 2
| Systems | Stimulation mechanisms | Cell types | Key results/advantages | Ref. |
|---|---|---|---|---|
| MEA | Electrical conduction measurement | nrCMs | Creation of cardiac muscle bundles and stem cell bridges using laser-guided patterning | Ma et al. (75) |
| PDMS microwells | Verify the electrical compatibility of different cell types | |||
| 3D aligned cardiac tissue bundles | Cx43 formation between stem cells and cardiac muscles | |||
| Flexible graphene MEA (G-MEA) | Electrical measurement | Cardiac-like HL-1 cells | Perform electrophysiological measurement, including beat rate, action potentials | Kireev et al. (76) |
| 2D culture | ||||
| 3D conductive material | Rectangular pulse 2 ms, 5 V/cm, 1 Hz | nrCMs | Produced thicker, intact and better aligned cardiac tissues | Dvir et al. (77) |
| Alginate scaffold | Induced synchronous beating | |||
| Gold nanowire | Increased Cx43 expression | |||
| Enhanced calcium transient | ||||
| 3D conductive material | Square pulse, 1 V/mm, 2 ms, 1Hz | nrCMs | Increased cell viability | Ganji et al. (78) |
| Polyurethane scaffold | Increased gene expression of Nkx2.5 ANF, and Mef2c | |||
| Gold nanotube and nanowire | Improved cell distribution and morphology | |||
| 3D bioreactor | Monophasic square pulse 3 V/cm, 3 Hz mechanical perfusion | nrCMs | Increased contraction amplitude | Maidhoff et al. (79) |
| Porous PGS scaffold | Improved DNA content and cell distribution | |||
| Carbone electrodes | Increased expression of cardiac markers (troponin I and troponin T) | |||
| Perfusion system | ||||
| 3D bioreactor | Rectangular pulse 2 ms, 5 V/cm, 1 Hz | nrCMs | Elongated and parallel-aligned cardiac tissues | Radisic et al. (80) |
| Ultra-foam collagen sponges placing on silicon spaces between two platinum electrodes | Higher contractile amplitude | |||
| Higher expression of cardiac markers (Cx43) | ||||
| Increase in MCR and decrease in ET | ||||
| 3D biowire in a PDMS template | Rectangular pulse, biphasic, 1 ms, 3–4 V/cm, 1–6 Hz | hESC/hiPSC-CMs | Increased MCR and CV | Nunes et al. (81) |
| Collagen gel | Decreased ET | |||
| Platinum wire coupled with carbon rods | Enhanced calcium handling | |||
| Enhanced myofibril ultrastructural organization | ||||
| 3D bioreactor | Rectanular pulse, biphasic, 1 ms, 3–4 V/cm, 1 Hz 5% mechanical static strain | nrCMs | Well-developed sarcomere structures | Miklas et al. (47) |
| PDMS tissue chamber with pneumatically driven stretching | Increased contractile forces | |||
| Carbon rods | Higher expression of Cs43, ANF and BNP | |||
| Collagen gel | Higher phosphorylation levels of ERK1/2 |
MEA, multi-electrode array; nrCMs, neonatal rat cardiomyocytes; PDMS, polydimethylsiloxane; MCR, maximum capture rate; ET, electrical threshold; CV, conduction velocity.
Electrical stimulation via MEA
MEAs have dual functions, as they can both stimulate CMs and measure their electrophysiological characteristics. In their simplest forms, MEAs are a planar array of biocompatible micro-transducers that act as ‘electrodes’ embedded in an insulating surface on which cells can be cultured directly (82). Since each electrode is electrically independent to its neighbors, multiple electrodes can be placed concurrently and micrometers apart, without electrical interference from neighboring electrodes (Figure 3A). The MEA can electrically stimulate the CMs, while enabling simultaneous localized recordings of extracellular field potentials for further analysis of beat rate, polarization behavior, and CV (86). Moreover, they are advantageous for long-term in vitro stem-cell derived CM studies, as they can noninvasively monitor the electrical activity.
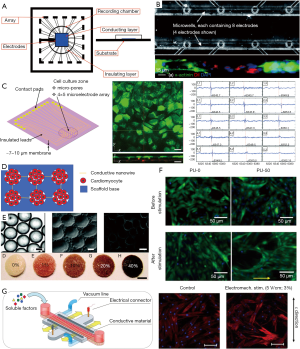
Ma et al. used MEA technology to validate a 3D cardiac muscle model intended to mimic stem cell transplantation in vitro (75). In this study, cardiac muscle model was established by creating eight parallel microwells on an MEA chip with each microwell containing eight electrodes (Figure 3B). nrCMs were seeded onto the MEA chip, cultured inside the microwells, and formed eight aligned cardiac muscle fibers. The center portion of each cardiac muscle fiber was removed to form a gap, where was then seeded with stem cells to form a “cell bridge”. MEAs were used to record the CV of the stem-cell bridges over several days. After two days, CV of the stem-cell bridges gradually increased to a stable value, which implied that cellular interaction with adjacent nrCMs could potentially regulate the stem cells to become more electrically compatible. Moreover, Cx43 was locally concentrated at the connection areas between stem-cell bridges and cardiac muscle fibers, which suggested the gap junctions might serve as the macromolecular channels for electrical propagation through the bridges. The use of MEA technology in this study was critical for validating the cardiac muscle model and verifying stem cell electrical compatibility when integrated into the damaged cardiac tissues.
A study by Kujala et al. investigated whether stimulation via an electrical field would enhance nrCM orientation, maturation and functional properties (87). In this study, nrCMs were cultured in the MEA chambers, in which two steel plate electrodes were placed 5 mm apart. Four experimental groups and an unstimulated control group were conducted with variation of pulse form (biphasic and monophasic), pulse duration (100 versus 2 ms), electrical field strength (5 and 2.5 V), duration of stimulation (2 versus 3 days), and culture material (2D coating of 0.1% gelatin versus 3D collagen gel). nrCMs under electrical stimulation showed no significant difference on viability, morphology, alignment and orientation compared to control groups. Although the beat rate of nrCMs was able to match the pacing frequency during electrical stimulation, it returned to original spontaneous frequency as the stimulation stopped, indicating that nrCMs did not maintain their paced rhythm after long-term electrical stimulation. This study proved the capability of stimulating nrCMs in the MEA chambers and measuring their electrical activity, though no long-term effects of electrical stimulation were observed on either morphological or electrophysiological behaviors of the nrCMs.
Currently, electrophysiological analyses of hiPSC-CMs have been confounded by biologically ill-defined factors, including the 3D spherical shape of the embryoid bodies, culture supplementation of animal serum, and feeder cells isolated from murine sources. Large variability in the aforementioned systems leads to uncontrollable and irreproducible results, making it difficult to develop conclusive studies on late stage maturation. A study by Zhu et al. used a chemically defined differentiation regimen, a monolayer cell culture of hESC-CMs, and serum-free culture media, and combined it with MEA technology to create a hybrid platform to accurately control and measure electrophysiological parameters of hESC-CMs (88). hESC-CMs cultured on the MEA platform achieved real-time, simultaneous acquisition of 120-channels of spatially distributed electrical data. Electrode channels underlying the cellular monolayer enabled examination of the electrophysiological maturation of hESC-CMs by monitoring the polarization and subsequent contractile behaviors from hESC-CMs over 10 days. Results showed that the hESC-CMs depolarization amplitude and the rate change in field potential progressively increased over the course of maturation. Moreover, the combined monolayer culture and MEA platform allowed for the identification of pacemaker cells and subsequent estimation of CV.
As MEA technology continues to progress, its’ utility in cardiac tissue engineering is becoming more widespread. Historically, in vitro MEA stimulation of engineered cardiac tissue requires at least 5 V to initiate tissue depolarization and pacing. However, these voltage levels far exceed the threshold voltage of water electrolysis (1.23 V), and produce gas bubbles in the culture medium that can impede nutrient stability and delivery to the tissues. Moreover, due to the high-glucose content of standard cardiac tissue culture media, electrical voltages in the range of 4–5 V can initiate redox reactions, which present risks of media pH shifts. To overcome these obstacles, Trada et al. designed and fabricated an implantable porous MEA (P-MEA) to function at low voltage, thereby mitigating voltage related risks of cell injury and undesirable oxidative byproducts (89). The P-MEA was embedded within the engineered cardiac tissues, thereby allowing for acute and long-term in vitro electrical stimulation at voltage levels below the thresholds that can result in cell injury and oxidative stress. The P-MEA successfully stimulated, paced and measured in the live cardiac tissues with a target voltage range between 0.35–0.42 V, with no indications of cellular stress. Additionally, there was a slight increase in the active contractile force as a response to the chronic pacing.
Recently, stretchable and flexible MEAs are being developed to perform dual electromechanical stimulation in vivo. Taylor et al. explored this concept by building a platform that applied cyclic mechanical strain and electrical stimulation to the CMs cultured on a stretchable MEA (90). The mechanical stretching was achieved by a serial-controlled linear actuator mounted onto a micromanipulator for uniaxial stretching cell substrates with a triangular waveform from 0–10% strain. The electrical pacing system was a modified commercial cell culture device providing a 10 V biphasic (10 ms high/10 ms low) waveform. CMs isolated from adult mice were seeded onto the MEA substrate and subjected to both mechanical stretching and electrical pacing at 1 Hz. This stretchable MEA platform coupling both electrical and mechanical stimulation induced natural electrophysiological properties on CMs, which strongly suggested that the combined effects promoted CM development and growth.
To expand the concept and application flexible MEA technology, graphene MEAs (G-MEAs) have been developed. The transparency and flexibility of graphene permits monitoring of cell viability and improves cellular contact and coupling. The high conductive properties of graphene allow it to be used actively as a transistor and passively as an electrode. Moreover, the G-MEAs offer ease of fabrication, in comparison with metal-based or other carbon-based MEA alternatives. Kireev et al. conducted a proof-of-concept study to ascertain if G-MEAs would provide reliable electrophysiological assessments of CM-like cells (76). The G-MEA chips were fabricated using transparent borofloat glass wafers. HL-1 cells were then cultured on top of the encapsulated G-MEA chips. Low noise recordings allowed for detection of cardiac extracellular field potentials with high signal-to-noise ratios. These experiments proved the applicability of the G-MEA for the complex electrophysiological recordings from networks of cells. To emphasize the versatility of graphene beyond G-MEAs, Wang et al. recently used biocompatible and superconductive graphene sheets to successfully induce the phenotypical transition of hiPSCs into functional CMs (91). The process increased the organization of the myofibril ultrastructure, CV, Ca2+ handling and Cx43 expression of the hiPSC-CMs. They reasoned that the graphene sheet acted as a conductive surface that mimicked the microenvironment of the heart, facilitating its intrinsic electrical propagation properties to promote the maturation of hiPSC-CMs.
To study myocytes and non-myocyte cells interactions based on the electrophysiological properties, a device called ‘perforated flexible MEA’ (PerFlexMEA) was developed to enable co-culturing nrCMs with non-myocyte cells (HeLa cells) on the two side of the MEA chip (83). The device consisted of an 8 µm thin parylene microporous membrane with 20 gold microelectrodes patterned on one side. The flexible parylene membrane was packaged between two rigid thermoplastic layers, such that only electrodes array region was exposed, while the rest of the device remained insulated. nrCMs and non-excitable HeLa cells were cultured on either side of the parylene membrane to create a tissue bilayer, and inter-layer electrical conduction can be measured by PerFlexMEA device (Figure 3C). The conduction velocity was observed to decrease after inducing lateral hetero-cellular controlled coupling between nrCMs and HeLa cells transfected by Cx43. PerFlexMEA was expected to allow studying the inter-layer electrical communications of bilayer tissue constructs, especially via direct contact formation of gap junctions.
Electrical stimulation via conductive scaffolds
Biomaterial scaffolds provide structural support to cellular matrices by providing a substrate to inoculate cells and mimicking features of natural ECMs. By customizing fabrication methods, materials and structural parameters, biomaterials scaffolds can be fine-tuned to optimize CM growth, and organize physiological relevant tissue structures that closely resemble native cardiac tissues. CMs cultured on conductive scaffolds (Figure 3D) and enriched via electrical stimulation have a higher potential for resolving into functional, transplantable tissues to replace damaged cardiac tissues (92). For efficient delivery of this stimulus, conductive polymers and conductive polymer composites are used as scaffold materials (93).
Park et al. explored the utility of a biomimetic scaffold combined with electrical stimulation and insulin-like growth factor-1 (IGF-1) (94). In this study, investigators designed a biodegradable PGS scaffold with anisotropic mechanical properties known to promote cardiac-like tissue structures. To these cell-laden constructs, both individual and combined effects of electrical stimulation (5 V/cm, 2 ms duration, 1 Hz) and IGF-1 (2 doses of 100 ng/mL) were evaluated. Three days after cell seeding, the scaffolds intended for electrical stimulation were fitted to carbon rod electrodes and oriented appropriately, such that the electrical field would run parallel with long pores of the scaffolds. After eight days, CMs supplemented with IGF-1 experienced a significant reduction in apoptosis as well as reduced ETs. With respect to tissue structure, the presence of stimulation, with or without IGF-1, was associated with the formation of tissue-like bundles oriented parallel to the direction of supplied electrical field. Electrically stimulated scaffolds also increased MMP-2 gene expression by more than tenfold with or without IGF-1. These observations implied that electrical stimulation promoted ECM remodeling in cardiac constructs, dissected tissue-like bundle formation, and increased MMP-2 gene expression. Higher Cx43 expression and more refined sarcomere development was found only with the supplementation of IGF-1 coupled with electrical stimulation. These results indicated a synergistic effect of supplemental IGF-1 and electrical stimulation on gap junction formation, sarcomere development, and an index of contractile activity. This study showed that the assembly of engineered heart tissue enabled by cardio-mimetic scaffolds could more closely recapitulate the in vivo developmental milieu through the interactive effects of biochemical factors and electrical stimulation.
3D nanocomposites of gold nanowires with macroporous alginate scaffolds were developed as conductive scaffolds to promote the cell-to-cell inter-connectivity with the absence of electrical stimulation. Dvir et al. compared the effects of gold nanowire-alginate composite scaffolds to a pristine alginate scaffolds on CM maturity after 3 days of culture (before electrical stimulation), and after 8 days of culture (5 days of post electrical stimulation with an AC electrical field) (77). As early as day 3, CMs within the nanowire scaffolds expressed higher levels of Cx43. By day 8, compared to the pristine alginate scaffolds, the nanowire-scaffolds produced thicker, intact, and better aligned cardiac tissues that were able to contract synchronously. Although many factors may have contributed to the improvement of tissue functionality via the nanowire scaffold, one possibility is that gold nanowires created conductive bridges across the alginate, thus connecting adjacent pores and cell bundles. Another possibility is that nanowires directly enhanced the expression of electrical coupling protein Cx43. The increase of tissue thickness could also play a role in the enhancement of electrical connectivity and functionality. Regardless, this work clearly illustrated how scaffold composites with conductive materials enhanced the scaffold functionality and cellular conductivity of CMs.
The biomaterial scaffolds used for cardiac tissue engineering were designed to couple electrical and elastic properties based on hydroxyethyl methacrylate (HEMA) hydrogel that was homogeneously dispersed with gold nanoparticles (Figure 3E). This conductive gel has Young’s moduli more similar to native myocardium relative to polyaniline and PPy. nrCMs were seeded to the conductive scaffolds and experienced with electrical stimulation at 2 mA rectangular pulses (2 ms, 1 Hz, 5 V/cm) for 5 days. The nrCMs were found to clustered within scaffold pores independent of the thiol-HEMA content and stimulation condition, while Cx43 expression was found to increase on the nrCMs under electrical stimulation (84). This novel scaffold provides a unique cellular microenvironment with tunable conductivity and elasticity, especially for cardiac tissue engineering.
Ganji et al. explored the concept of culturing cardiac patches on porous, biodegradable polyurethane (PU) scaffold substrates that could be directly implanted onto infarcted tissues (78). Gold nanotubes/nanowires (PU-GNT/NW) were incorporated into the PU scaffolds to mimic the electromechanical properties of the myocardium. The PU-GNT/NW composites were evaluated at three composite levels: PU-0 for the scaffolds containing no GNT/NW; PU-50 for the scaffolds containing 50 ppm GNT/NW; PU-100 for the scaffolds containing 100 ppm. H9C2 rat CMs were seeded on the conductive PU scaffolds, and then conditioned with 15 minutes of electrical stimulation (1 V/mm, 2 ms, 1 Hz) for three consecutive days. After 1 day of incubation, cells were more homogeneously distributed within the PU-50 scaffolds than on the other PU-0 or PU-100 scaffolds. It was shown that cell alignment was apparent on only gold containing scaffolds, but not on the PU-0 scaffolds. Cell confluency before and after electrical stimulation demonstrated a significant improvement of 39% in the PU-50 samples and a slight of 14% in the PU-100 samples. The gold/PU nanocomposite scaffolds significantly increased the gene expression level of early cardiac transcription factors Nkx2.5 and Mef2c. The improvements in cell morphology and gene expression illustrated that the incorporation of conductive materials, such as gold, can significantly promote CM maturity and functionality (Figure 3F).
3D printing technology has offered the unprecedented ability to fabricate 3D scaffolds and maintain precise control over the scaffold architecture. 3D printing allows consistency in pore size, pore geometry and mechanical strength, which is advantageous for larger scale production. Adams et al. used 3D printed scaffolds to determine a suitable scaffold material and assess how electrical stimulation influenced CM development (95). Individual scaffolds of PCL and silicone rubber were printed with identical porosity and strand size, and seeded with adult primary human CMs. The percentage of CMs attached in the PCL scaffolds relative to the number of cells seeded was significantly greater (41%) than silicone rubber scaffolds (5.3%), indicating that PCL was more compatible for CM attachment and growth. CMs cultured on PCL were subjected to 3 hours of electrical stimulation (5 V, 2 ms, 1 Hz) using a wireless control system that did not disturb the culture environment. Confocal images of both stimulated and unstimulated cultures showed that cells had successfully attached to the scaffolds. However, bright field micrographs showed that on the scaffolds subjected to electrical stimulation, cell density was higher and more closely aggregated towards the scaffold strands than unstimulated cultures. This demonstrated that electrical stimulation improved the cellular compatibility of PCL scaffolds.
Gelmi et al. used an electromechanically active fiber scaffold to provide electrical and mechanical stimulation for the differentiation of hiPSC-CMs (96). PLGA was selected as the scaffold material because of non-conductive, biocompatible properties and the ability to easily fabricate via electrospinning. The PLGA fibers were coated with PPy through vapor phase polymerization. Following, an electrochemical polymerization process was then used to coat the fibers in an aqueous pyrrole monomer solution to produce a thick, more controlled layer of PPy. hiPSC-CMs seeded on PLGA-PPy fibers stimulated for maximum mechanical actuation showed aligned morphology and elevated expression of cardiac markers actinin, NKX2.5, GATA4, compared to those on plain PLGA.
Though scaffold technology is progressing rapidly, these microstructures are vast simplifications of the complex in vivo ECM environment. One disadvantage of using scaffolds is cell leakage that often occurs during cell injection into the porous structure of the acellular scaffolds. Because of the complexity of native tissue, most approaches are limited to studying one or two aspects of tissue regeneration at a time. However, to mimic the in vivo environment, these systems need to attend to physical structure, functionality, vascularization, and supply simultaneous electromechanical stimulation (97). Since different aspects of the 3D scaffold designs may conflict with each other, addressing all the needs for engineering cardiac tissues is extremely challenging. The optimization of scaffold designs, materials, and stimulation parameters of scaffolds holds incredible potentials for cardiac tissue engineering.
Electrical stimulation via bioreactors
To overcome the limitations of MEAs and conductive scaffolds, bioreactors are being extensively developed with more sophisticated design parameters. Bioreactors are apparatuses used to study biological reactions in an environmentally controlled system. Bioreactors allow clinically sustainable biological processes to be studied in an automated, repeatable and scalable manner, particularly for cell expansion, differentiation and tissue maturation (98). In tissue engineering, a bioreactor simulates the in vivo signaling environment by applying electrical, mechanical and/or chemical stimuli to aid in promoting cell differentiation and ECM production in vitro (99). Bioreactors can be designed to provide spatially uniform cell distribution, maintain the desired concentration of gases and nutrients in culture medium, and induce mass transport to the tissue, and facilitate the formation of 3D tissues (100,101). Bioreactors have also been designed to deliver electrical stimuli to the cardiac cells or tissues for a long-term stimulation (about 6 weeks) with stable properties over a wide range of input amplitudes and frequencies (98).
Taking advantage of the controlled tissue culture conditions supplied by the bioreactors, Maidhoff et al. investigated the synergistic effects of nutrient perfusion, electrical stimulation and unconstrained contraction of the cardiac constructs (79). To protect CMs from shear flow, a porous PGS scaffold was used as a substrate for homogeneously seeding CMs. This porous substrate in the bioreactor allowed medium to flow through the tissue construct without any external fixation, thus mimicking the dense capillary network for blood flows through the myocardium. Then, an electrical field was generated by two parallel carbon electrodes attached to the cardiac stimulator that provided repeated electrical pulses (3 V/cm, 3 Hz, monophasic square wave). An enhancement of contractile capability of the cardiac constructs was observed, such as a 0.23%±0.10% increased contraction amplitude of stimulated/perfused cardiac constructs in comparison to the control constructs. Stimulated/perfused constructs also exhibited improved DNA contents, better cell distribution throughout the scaffold thickness, higher cardiac gene expression, as well as physiological cell morphology and tissue organization. However, this model did not fully represent uniformly interconnected tissue structure, due to the dense clusters formed by cardiac cells throughout tissues.
To evaluate the optimal time scale of cardiac construct stimulation, Radisic et al. developed a bioreactor system to culture and stimulate the nrCMs seeded onto ultra-foam collagen sponges (80). The electrical mechanism of the bioreactor, consisting of six tissue culture wells—each containing one cardiac construct, was constructed by placing silicon spacers between two electrodes. Platinum wires connected a cardiac stimulator to carbon electrodes, which supplied trains of electrical pulses (rectangular, 2 ms, 5 V/cm, 1 Hz) to the cardiac constructs. This was intended to replicate the electrophysiological characteristics of the native myocardium. After 8 days of culture, the electrically stimulated cardiac constructs showed a decrease in ET and an increase in MCR. Stimulated constructs also exhibited elongated and parallel-aligned tissue morphology that closely resembled the native myocardium. More importantly, the extent of the beneficial effects of electrical stimulation depended heavily on the time of initiation compared to the time in culture. Both premature stimulation (one day after cell seeding) and late stimulation (five days after cell seeding) failed to enhance the functional tissue organization and contractile synchronicity. The optimal time for electrical stimulation was found to be 3 days after cell seeding, which resulted in, relative to the unstimulated constructs, a high contractile amplitude (7-fold higher) and elevated levels of cardiac markers (e.g., Cx43). Because of the high-controlled conditions in the bioreactors, they could systematically determine the optimal time to initiate electrical pulses to induce maturity of the tissue constructs.
As a microscale derivative of bioreactors for electrical stimulation, biowires mimic 3D native cardiac tissue bundles by casting hiPSC-CMs with collagen gel in microfabricated wells and applying electrical stimulation. Nunes et al. demonstrated that biowires started spontaneously beating 2–3 days after gel compaction (81). After pre-culturing hESC-CMs for 1 week, the biowires were electrically stimulated for 7 days, and at increasing increments either from 1–3 Hz or from 1–6 Hz. The biowires, particularly in the ones with 6 Hz stimulation, exhibited organized sarcomeric banding and myofibrils that converged the aligned Z discs. The stimulated biowires also exhibited lower ET, higher MCR, higher CV and improved electrophysiological and Ca2+ handling properties. It was also observed improved hESC-CMs architecture, increased sarcomere maturation, and enhanced electrophysiological properties in a stimulation frequency dependent manner (102). Xiao et al. further improved the biowire design for in vitro cardiac pharmacology studies by adding perfusion capabilities for high throughput drug screening (103).
To further explore the synergistic effects of various forms of stimulation, Pavesi et al. developed a bioreactor that allowed for simultaneous electrical, mechanical and biochemical stimulation, thereby more accurately recapitulating the complex in vivo conditions (85). The bioreactor was designed to have three functional layers so that each stimulus could be applied independently from the other. PDMS was chosen to apply mechanical stimulation in the pneumatic layer. For the conductive layer, PDMS pre-polymer was doped with carbon nanotubes to deliver electrical stimulation. The final design had thin deformable lateral walls, through which negative pressure from side channels would apply uniform strain to the cells on the membranes (Figure 3G). Human mesenchymal stem cells were seeded onto the bioreactors as six experimental cohorts: a control cohort without mechanical or electrical stimulation, two mechanical cohorts with either a 3% or 7% strain, an electrical cohort with an electric field of 5 V/cm via biphasic square-wave pulses (+/− 1.2 V for 1 ms), and two electromechanical cohorts with electrical stimulation coupled with either 3% or 7% mechanical strain. This study found statistically significant differences in Cx43 fluorescence intensities between the control and the electromechanically stimulated groups at the lower strain levels (3%). Under only 3% mechanical strain, the CMs displayed actin fibers oriented perpendicular to the direction of strain. In addition, the intensities of Cx43 showed statistically significant differences between control samples and samples subjected to 5 V/cm electrical stimulation and 3% strain. The study demonstrated that this novel micro-bioreactor technology was capable of providing controlled electrical, mechanical and biochemical stimulations to cell cultures to assess the impact of individual and combinatorial stimuli on stem cell development.
Miklas et al. developed a platform that combined electrical and mechanical stimulation to cardiac microtissues (47). The bioreactor consisted of a PDMS chamber capable of holding eight microtissues attached to the stimulatory electrodes and placed in a custom-made stretching platform. Each well had posts that acted as a fixation point for CMs and measured the contraction force based on a beam-deflection model. The nrCM microtissues were then subjected to both electrical stimulation and mechanical stimulation. While there was no significant difference in ET and MCR of the different groups, well-developed sarcomere structures and increased contraction force were observed in the group subjected to electromechanical stimulation compared to those with single stimulation or no stimulation. Static strain at 5% resulted in poor formation of myofibrils with some aligning perpendicular to the direction of strain. There was also no significant difference in the expression of SERCA, while higher expression was found on Cx43, ANF and BNP in the 5% strain + 1 Hz groups. However, the levels of ERK1/2 and phosphorylation levels of ERK1/2, both associated with physiological hypertrophy, were comparable in the three groups.
Research in bioreactor engineering has shown to be extremely diverse and versatile. Although this immense variety of bioreactors has demonstrated the potential in cardiac tissue engineering, there are still many challenges that need to be addressed in order to replicate the complex in vivo conditions, including vascularization and supply of oxygen and soluble nutrients to in vitro culture of 3D thick tissues. For the materials used in the bioreactors, despite the versatile material selections based on different applications, silicon polymer and other typical microfabricated substrates were commonly used. However, these materials are orders of magnitude stiffer than typical biological tissues, which can heavily impact differentiation, proliferation, alignment, and function of the cardiovascular cells. Thus, design and fabrication of new conductive materials for creating physiological relevant cardiac tissues are the priority in the development of next generation bioreactors for electrical stimulation. Future bioreactors should also allow for real-time monitoring of the cardiac constructs responding to specific conditions, and further adjusting the culture parameters adaptive to the maturation phase. Additionally, conventional bioreactors require large operating volumes, which is critical for the large consumption of expensive media components. To reach clinical efficacy, a more cost-effective manufacturing process should be developed for the future bioreactor design.
Conclusions
In vivo cardiac environments manifest complexities that have proven to be a challenge to replicate in vitro. This is because cardiac tissues are simultaneously subjected to numerous stimuli, including shear stress from blood flow through the myocardium, compression and stretching stress from contractile forces, and intricately paced electrical bio-circuitry. To this end, researchers are constantly optimizing CM culture in an in vitro microenvironment that promotes differentiation and maturation with the overall goal of recapitulating cardiac functions. This challenge has been partially addressed with the rapid development of cardiac tissue microsystems, which have been engineered to supply biophysical, electrical and biochemical cues to accelerate tissue maturation. However, with each new system deviating further away from traditional tissue culture environments, we face challenges in biocompatibility as well as characterizing tissue physiology on these new devices. Despite these challenges, creative designs and innovative methods can revolutionize how we approach tissue engineering problems.
This review investigated the impact of mechanical stimulation on CM development and maturity. To summarize, many studies have determined that intermittent or cyclical stimulation may improve the proliferation and morphology of CMs and their contractile strength, emphasizing the importance mechanical loading on tissue development and function. Additionally, in vitro electrical stimulation of CMs was shown to enhance cellular alignment in the direction of the electric field, promote CM maturation, and regulate cardio-mimetic contractile synchronicity. While some groups have studied these stimulatory cues individually, researchers are now focusing on the synergistic effects of mechanical stimulation coupled with electrical stimulation to unveil novel biological findings. With new technologies in microfabrication, 3D printing and bioreactor engineering, these microsystems show great potential for the future of cardiac tissue engineering and regenerative medicine.
Acknowledgments
Funding: This work was supported by the Nappi Family Foundation Research Scholar Project and SU Collaboration for Unprecedented Success and Excellence (CUSE) Grant. M.Z. acknowledges support from National Science Foundation (NSF-EBMS-1804875), Lush Prize Young Researchers at Americas. P.H. acknowledges support from the National Science Foundation Integrative Graduate Education and Research Traineeship (NSF IGERT), DMR-DGE-1068780.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/mps.2018.11.01). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Brette F, Orchard C. T-tubule function in mammalian cardiac myocytes. Circ Res 2003;92:1182-92. [Crossref] [PubMed]
- Louch WE, Bito V, Heinzel FR, et al. Reduced synchrony of Ca2+ release with loss of T-tubules-a comparison to Ca2+ release in human failing cardiomyocytes. Cardiovasc Res 2004;62:63-73. [Crossref] [PubMed]
- Martin-Rendon E, Brunskill SJ, Hyde CJ, et al. Autologous bone marrow stem cells to treat acute myocardial infarction: a systematic review. Eur Heart J 2008;29:1807-18. [Crossref] [PubMed]
- Abdel-Latif A, Bolli R, Tleyjeh IM, et al. Adult bone marrow-derived cells for cardiac repair: a systematic review and meta-analysis. Arch Intern Med 2007;167:989-97. [Crossref] [PubMed]
- Chen SL, Fang WW, Ye F, et al. Effect on left ventricular function of intracoronary transplantation of autologous bone marrow mesenchymal stem cell in patients with acute myocardial infarction. Am J Cardiol 2004;94:92-5. [Crossref] [PubMed]
- Agarwal A, Goss JA, Cho A, et al. Microfluidic heart on a chip for higher throughput pharmacological studies. Lab Chip 2013;13:3599-608. [Crossref] [PubMed]
- Bhatia SN, Ingber DE. Microfluidic organs-on-chips. Nat Biotechnol 2014;32:760-72. [Crossref] [PubMed]
- Wikswo JP. The relevance and potential roles of microphysiological systems in biology and medicine. Exp Biol Med (Maywood) 2014;239:1061-72. [Crossref] [PubMed]
- Ma Z, Koo S, Finnegan MA, et al. Three-dimensional filamentous human diseased cardiac tissue model. Biomaterials 2014;35:1367-77. [Crossref] [PubMed]
- Huh D, Matthews BD, Mammoto A, et al. Reconstituting organ-level lung functions on a chip. Science 2010;328:1662-8. [Crossref] [PubMed]
- Kim HJ, Huh D, Hamilton G, et al. Human gut-on-a-chip inhabited by microbial flora that experiences intestinal peristalsis-like motions and flow. Lab Chip 2012;12:2165-74. [Crossref] [PubMed]
- Mathur A, Loskill P, Shao K, et al. Human iPSC-based cardiac microphysiological system for drug screening applications. Sci Rep 2015;5:8883. [Crossref] [PubMed]
- Jastrzebska E, Tomecka E, Jesion I. Heart-on-a-chip based on stem cell biology. Biosens Bioelectron 2016;75:67-81. [Crossref] [PubMed]
- Halldorsson S, Lucumi E, Gomez-Sjoberg R, et al. Advantages and challenges of microfluidic cell culture in polydimethylsiloxane devices. Biosens Bioelectron 2015;63:218-31. [Crossref] [PubMed]
- Young EW, Beebe DJ. Fundamentals of microfluidic cell culture in controlled microenvironments. Chem Soc Rev 2010;39:1036-48. [Crossref] [PubMed]
- Ertl P, Sticker D, Charwat V, et al. Lab-on-a-chip technologies for stem cell analysis. Trends Biotechnol 2014;32:245-53. [Crossref] [PubMed]
- Gobaa S, Hoehnel S, Roccio M, et al. Artificial niche microarrays for probing single stem cell fate in high throughput. Nat Methods 2011;8:949-55. [Crossref] [PubMed]
- Engler AJ, Sen S, Sweeney HL, et al. Matrix elasticity directs stem cell lineage specification. Cell 2006;126:677-89. [Crossref] [PubMed]
- Wolfram CJ, Rubloff GW, Luo X. Perspectives in flow-based microfluidic gradient generators for characterizing bacterial chemotaxis. Biomicrofluidics 2016;10:061301 [Crossref] [PubMed]
- Yim EK, Darling EM, Kulangara K, et al. Nanotopography-induced changes in focal adhesions, cytoskeletal organization, and mechanical properties of human mesenchymal stem cells. Biomaterials 2010;31:1299-306. [Crossref] [PubMed]
- Robertson C, Tran DD, George SC. Concise review: maturation phases of human pluripotent stem cell-derived cardiomyocytes. Stem Cells 2013;31:829-37. [Crossref] [PubMed]
- Mummery CL, Zhang J, Ng ES, et al. Differentiation of human embryonic stem cells and induced pluripotent stem cells to cardiomyocytes: a methods overview. Circ Res 2012;111:344-58. [Crossref] [PubMed]
- Baharvand H, Piryaei A, Rohani R, et al. Ultrastructural comparison of developing mouse embryonic stem cell- and in vivo-derived cardiomyocytes. Cell Biol Int 2006;30:800-7. [Crossref] [PubMed]
- Jozefczuk J, Prigione A, Chavez L, et al. Comparative analysis of human embryonic stem cell and induced pluripotent stem cell-derived hepatocyte-like cells reveals current drawbacks and possible strategies for improved differentiation. Stem Cells Dev 2011;20:1259-75. [Crossref] [PubMed]
- Li Z, Hu S, Ghosh Z, et al. Functional characterization and expression profiling of human induced pluripotent stem cell- and embryonic stem cell-derived endothelial cells. Stem Cells Dev 2011;20:1701-10. [Crossref] [PubMed]
- Shadrin IY, Allen BW, Qian Y, et al. Cardiopatch platform enables maturation and scale-up of human pluripotent stem cell-derived engineered heart tissues. Nat Commun 2017;8:1825. [Crossref] [PubMed]
- Godier-Furnémont AF, Tiburcy M, Wagner E, et al. Physiologic force-frequency response in engineered heart muscle by electromechanical stimulation. Biomaterials 2015;60:82-91. [Crossref] [PubMed]
- Endoh M. Force-frequency relationship in intact mammalian ventricular myocardium: physiological and pathophysiological relevance. Eur J Pharmacol 2004;500:73-86. [Crossref] [PubMed]
- Ziman AP, Gomez-Viquez NL, Bloch RJ, et al. Excitation-contraction coupling changes during postnatal cardiac development. J Mol Cell Cardiol 2010;48:379-86. [Crossref] [PubMed]
- Bers DM. Cardiac excitation-contraction coupling. Nature 2002;415:198-205. [Crossref] [PubMed]
- Bers DM. Sarcoplasmic reticulum Ca release in intact ventricular myocytes. Front Biosci 2002;7:d1697-711. [Crossref] [PubMed]
- Vornanen M. Excitation-contraction coupling of the developing rat heart. Mol Cell Biochem 1996;163-164:5-11. [Crossref] [PubMed]
- Germanguz I, Sedan O, Zeevi-Levin N, et al. Molecular characterization and functional properties of cardiomyocytes derived from human inducible pluripotent stem cells. J Cell Mol Med 2011;15:38-51. [Crossref] [PubMed]
- Binah O, Dolnikov K, Sadan O, et al. Functional and developmental properties of human embryonic stem cells-derived cardiomyocytes. J Electrocardiol 2007;40:S192-6. [Crossref] [PubMed]
- Dolnikov K, Shilkrut M, Zeevi-Levin N, et al. Functional properties of human embryonic stem cell-derived cardiomyocytes: intracellular Ca2+ handling and the role of sarcoplasmic reticulum in the contraction. Stem Cells 2006;24:236-45. [Crossref] [PubMed]
- Zeevi-Levin N, Itskovitz-Eldor J, Binah O. Functional properties of human embryonic stem cell-derived cardiomyocytes. Crit Rev Eukaryot Gene Expr 2010;20:51-9. [Crossref] [PubMed]
- Kolanowski TJ, Antos CL, Guan K. Making human cardiomyocytes up to date: Derivation, maturation state and perspectives. Int J Cardiol 2017;241:379-86. [Crossref] [PubMed]
- Yang X, Pabon L, Murry CE. Engineering adolescence: maturation of human pluripotent stem cell-derived cardiomyocytes. Circ Res 2014;114:511-23. [Crossref] [PubMed]
- Bedada FB, Wheelwright M, Metzger JM. Maturation status of sarcomere structure and function in human iPSC-derived cardiac myocytes. Biochim Biophys Acta 2016;1863:1829-38. [Crossref] [PubMed]
- Ronaldson-Bouchard K, Ma SP, Yeager K, et al. Advanced maturation of human cardiac tissue grown from pluripotent stem cells. Nature 2018;556:239-43. [Crossref] [PubMed]
- Zhang B, Montgomery M, Chamberlain MD, et al. Biodegradable scaffold with built-in vasculature for organ-on-a-chip engineering and direct surgical anastomosis. Nat Mater 2016;15:669-78. [Crossref] [PubMed]
- Vollert I, Seiffert M, Bachmair J, et al. In vitro perfusion of engineered heart tissue through endothelialized channels. Tissue Eng Part A 2014;20:854-63. [PubMed]
- Zhang YS, Arneri A, Bersini S, et al. Bioprinting 3D microfibrous scaffolds for engineering endothelialized myocardium and heart-on-a-chip. Biomaterials 2016;110:45-59. [Crossref] [PubMed]
- Stoehr A, Hirt MN, Hansen A, et al. Spontaneous Formation of Extensive Vessel-Like Structures in Murine Engineered Heart Tissue. Tissue Eng Part A 2016;22:326-35. [Crossref] [PubMed]
- Ruwhof C, van der Laarse A. Mechanical stress-induced cardiac hypertrophy: mechanisms and signal transduction pathways. Cardiovasc Res 2000;47:23-37. [Crossref] [PubMed]
- Sadoshima J, Jahn L, Takahashi T, et al. Molecular characterization of the stretch-induced adaptation of cultured cardiac cells. An in vitro model of load-induced cardiac hypertrophy. J Biol Chem 1992;267:10551-60. [PubMed]
- Miklas JW, Nunes SS, Sofla A, et al. Bioreactor for modulation of cardiac microtissue phenotype by combined static stretch and electrical stimulation. Biofabrication 2014;6:024113 [Crossref] [PubMed]
- Salameh A, Wustmann A, Karl S, et al. Cyclic mechanical stretch induces cardiomyocyte orientation and polarization of the gap junction protein connexin43. Circ Res 2010;106:1592-602. [Crossref] [PubMed]
- Zhuang J, Yamada KA, Saffitz JE, et al. Pulsatile stretch remodels cell-to-cell communication in cultured myocytes. Circ Res 2000;87:316-22. [Crossref] [PubMed]
- Sidorov VY, Samson PC, Sidorova TN, et al. I-Wire Heart-on-a-Chip I: Three-dimensional cardiac tissue constructs for physiology and pharmacology. Acta Biomater 2017;48:68-78. [Crossref] [PubMed]
- Ruan JL, Tulloch NL, Saiget M, et al. Mechanical Stress Promotes Maturation of Human Myocardium From Pluripotent Stem Cell-Derived Progenitors. Stem Cells 2015;33:2148-57. [Crossref] [PubMed]
- Shachar M, Benishti N, Cohen S. Effects of mechanical stimulation induced by compression and medium perfusion on cardiac tissue engineering. Biotechnol Prog 2012;28:1551-9. [Crossref] [PubMed]
- Giridharan GA, Nguyen MD, Estrada R, et al. Microfluidic cardiac cell culture model (muCCCM). Anal Chem 2010;82:7581-7. [Crossref] [PubMed]
- Marsano A, Conficconi C, Lemme M, et al. Beating heart on a chip: a novel microfluidic platform to generate functional 3D cardiac microtissues. Lab Chip 2016;16:599-610. [Crossref] [PubMed]
- Norman JJ, Mukundan V, Bernstein D, et al. Microsystems for biomechanical measurements. Pediatr Res 2008;63:576-83. [Crossref] [PubMed]
- Yin S, Zhang X, Zhan C, et al. Measuring single cardiac myocyte contractile force via moving a magnetic bead. Biophys J 2005;88:1489-95. [Crossref] [PubMed]
- Beussman KM, Rodriguez ML, Leonard A, et al. Micropost arrays for measuring stem cell-derived cardiomyocyte contractility. Methods 2016;94:43-50. [Crossref] [PubMed]
- Turnbull IC, Karakikes I, Serrao GW, et al. Advancing functional engineered cardiac tissues toward a preclinical model of human myocardium. FASEB J 2014;28:644-54. [Crossref] [PubMed]
- Grosberg A, Alford PW, McCain ML, et al. Ensembles of engineered cardiac tissues for physiological and pharmacological study: heart on a chip. Lab Chip 2011;11:4165-73. [Crossref] [PubMed]
- Schaaf S, Shibamiya A, Mewe M, et al. Human engineered heart tissue as a versatile tool in basic research and preclinical toxicology. PLoS One 2011;6:e26397 [Crossref] [PubMed]
- Boudou T, Legant WR, Mu A, et al. A microfabricated platform to measure and manipulate the mechanics of engineered cardiac microtissues. Tissue Eng Part A 2012;18:910-9. [Crossref] [PubMed]
- Hinson JT, Chopra A, Nafissi N, et al. HEART DISEASE. Titin mutations in iPS cells define sarcomere insufficiency as a cause of dilated cardiomyopathy. Science 2015;349:982-6. [Crossref] [PubMed]
- Wang G, McCain ML, Yang L, et al. Modeling the mitochondrial cardiomyopathy of Barth syndrome with induced pluripotent stem cell and heart-on-chip technologies. Nat Med 2014;20:616-23. [Crossref] [PubMed]
- Lee YU, Hayman D, Sprague EA, et al. Effects of Axial Stretch on Cell Proliferation and Intimal Thickness in Arteries in Organ Culture. Cell Mol Bioeng 2010;3:286-95. [Crossref] [PubMed]
- Benhardt HA, Cosgriff-Hernandez EM. The role of mechanical loading in ligament tissue engineering. Tissue Eng Part B Rev 2009;15:467-75. [Crossref] [PubMed]
- Powell HM, McFarland KL, Butler DL, et al. Uniaxial strain regulates morphogenesis, gene expression, and tissue strength in engineered skin. Tissue Eng Part A 2010;16:1083-92. [Crossref] [PubMed]
- Lee WC, Maul TM, Vorp DA, et al. Effects of uniaxial cyclic strain on adipose-derived stem cell morphology, proliferation, and differentiation. Biomech Model Mechanobiol 2007;6:265-73. [Crossref] [PubMed]
- DuFort CC, Paszek MJ, Weaver VM. Balancing forces: architectural control of mechanotransduction. Nat Rev Mol Cell Biol 2011;12:308-19. [Crossref] [PubMed]
- Holle AW, Engler AJ. More than a feeling: discovering, understanding, and influencing mechanosensing pathways. Curr Opin Biotechnol 2011;22:648-54. [Crossref] [PubMed]
- Schroer AK, Shotwell MS, Sidorov VY, et al. I-Wire Heart-on-a-Chip II: Biomechanical analysis of contractile, three-dimensional cardiomyocyte tissue constructs. Acta Biomater 2017;48:79-87. [Crossref] [PubMed]
- Tzatzalos E, Abilez OJ, Shukla P, et al. Engineered heart tissues and induced pluripotent stem cells: Macro- and microstructures for disease modeling, drug screening, and translational studies. Adv Drug Deliv Rev 2016;96:234-44. [Crossref] [PubMed]
- Liaw NY, Zimmermann WH. Mechanical stimulation in the engineering of heart muscle. Adv Drug Deliv Rev 2016;96:156-60. [Crossref] [PubMed]
- Jackman CP, Carlson AL, Bursac N. Dynamic culture yields engineered myocardium with near-adult functional output. Biomaterials 2016;111:66-79. [Crossref] [PubMed]
- Mathur A, Ma Z, Loskill P, et al. In vitro cardiac tissue models: Current status and future prospects. Adv Drug Deliv Rev 2016;96:203-13. [Crossref] [PubMed]
- Ma Z, Liu Q, Liu H, et al. Laser-patterned stem-cell bridges in a cardiac muscle model for on-chip electrical conductivity analyses. Lab Chip 2012;12:566-73. [Crossref] [PubMed]
- Kireev D, Seyock S, Lewen J, et al. Graphene Multielectrode Arrays as a Versatile Tool for Extracellular Measurements. Adv Healthc Mater 2017;6. [PubMed]
- Dvir T, Timko BP, Brigham MD, et al. Nanowired three-dimensional cardiac patches. Nat Nanotechnol 2011;6:720-5. [Crossref] [PubMed]
- Ganji Y, Li Q, Quabius ES, et al. Cardiomyocyte behavior on biodegradable polyurethane/gold nanocomposite scaffolds under electrical stimulation. Mater Sci Eng C Mater Biol Appl 2016;59:10-8. [Crossref] [PubMed]
- Maidhof R, Tandon N, Lee EJ, et al. Biomimetic perfusion and electrical stimulation applied in concert improved the assembly of engineered cardiac tissue. J Tissue Eng Regen Med 2012;6:e12-23. [Crossref] [PubMed]
- Radisic M, Park H, Shing H, et al. Functional assembly of engineered myocardium by electrical stimulation of cardiac myocytes cultured on scaffolds. Proc Natl Acad Sci U S A 2004;101:18129-34. [Crossref] [PubMed]
- Nunes SS, Miklas JW, Liu J, et al. Biowire: a platform for maturation of human pluripotent stem cell-derived cardiomyocytes. Nat Methods 2013;10:781-7. [Crossref] [PubMed]
- Jones IL, Livi P, Lewandowska MK, et al. The potential of microelectrode arrays and microelectronics for biomedical research and diagnostics. Anal Bioanal Chem 2011;399:2313-29. [Crossref] [PubMed]
- Mondal A, Baker B, Harvey IR, et al. PerFlexMEA: a thin microporous microelectrode array for in vitro cardiac electrophysiological studies on hetero-cellular bilayers with controlled gap junction communication. Lab Chip 2015;15:2037-48. [Crossref] [PubMed]
- You JO, Rafat M, Ye GJ, et al. Nanoengineering the heart: conductive scaffolds enhance connexin 43 expression. Nano Lett 2011;11:3643-8. [Crossref] [PubMed]
- Pavesi A, Adriani G, Rasponi M, et al. Controlled electromechanical cell stimulation on-a-chip. Sci Rep 2015;5:11800. [Crossref] [PubMed]
- Reppel M, Pillekamp F, Brockmeier K, et al. The electrocardiogram of human embryonic stem cell-derived cardiomyocytes. J Electrocardiol 2005;38:166-70. [Crossref] [PubMed]
- Kujala K, Ahola A, Pekkanen-Mattila M, et al. Electrical Field Stimulation with a Novel Platform: Effect on Cardiomyocyte Gene Expression but not on Orientation. Int J Biomed Sci 2012;8:109-20. [PubMed]
- Zhu H, Scharnhorst KS, Stieg AZ, et al. Two dimensional electrophysiological characterization of human pluripotent stem cell-derived cardiomyocyte system. Sci Rep 2017;7:43210. [Crossref] [PubMed]
- Tradal HV, Vendra V, Tinney JP, et al. Implantable Thin-film Porous Microelectrode Array (P-MEA) for Electrical Stimulation of Engineered Cardiac Tissues. Biochip J 2015;9:85-96. [Crossref]
- Taylor RE, Boyce CM, Boyce MC, et al. Planar patterned stretchable electrode arrays based on flexible printed circuits. J Micromech Microeng 2013;23. [PubMed]
- Wang J, Cui C, Nan H, et al. Graphene Sheet-Induced Global Maturation of Cardiomyocytes Derived from Human Induced Pluripotent Stem Cells. ACS Appl Mater Interfaces 2017;9:25929-40. [Crossref] [PubMed]
- Wang B, Wang GJ, To F, et al. Myocardial Scaffold-Based Cardiac Tissue Engineering: Application of Coordinated Mechanical and Electrical Stimulations. Langmuir 2013;29:11109-17. [Crossref] [PubMed]
- Balint R, Cassidy NJ, Cartmell SH. Conductive polymers: Towards a smart biomaterial for tissue engineering. Acta Biomaterialia 2014;10:2341-53. [Crossref] [PubMed]
- Park H, Larson BL, Kolewe ME, et al. Biomimetic scaffold combined with electrical stimulation and growth factor promotes tissue engineered cardiac development. Exp Cell Res 2014;321:297-306. [Crossref] [PubMed]
- Adams SD, Ashok A, Kanwar RK, et al. Integrated 3D printed scaffolds and electrical stimulation for enhancing primary human cardiomyocte cultures. Bioprinting 2017;6:18-24. [Crossref]
- Gelmi A, Cieslar-Pobuda A, de Muinck E, et al. Direct Mechanical Stimulation of Stem Cells: A Beating Electromechanically Active Scaffold for Cardiac Tissue Engineering. Adv Healthc Mater 2016;5:1471-80. [Crossref] [PubMed]
- Tandon N, Marolt D, Cimetta E, et al. Bioreactor engineering of stem cell environments. Biotechnol Adv 2013;31:1020-31. [Crossref] [PubMed]
- Massai D, Cerino G, Gallo D, et al. Bioreactors as Engineering Support to Treat Cardiac Muscle and Vascular Disease. J Healthc Eng 2013;4:329-70. [Crossref] [PubMed]
- Darling EM, Athanasiou KA. Articular cartilage Bioreactors and bioprocesses. Tissue Eng 2003;9:9-26. [Crossref] [PubMed]
- Vunjak-Novakovic G, Obradovic B, Martin I, et al. Dynamic cell seeding of polymer scaffolds for cartilage tissue engineering. Biotechnol Prog 1998;14:193-202. [Crossref] [PubMed]
- Bancroft GN, Sikavitsas VI, Mikos AG. Design of a flow perfusion bioreactor system for bone tissue-engineering applications. Tissue Eng 2003;9:549-54. [Crossref] [PubMed]
- Sun X, Nunes SS. Biowire platform for maturation of human pluripotent stem cell-derived cardiomyocytes. Methods 2016;101:21-6. [Crossref] [PubMed]
- Xiao Y, Zhang BY, Liu HJ, et al. Microfabricated perfusable cardiac biowire: a platform that mimics native cardiac bundle. Lab Chip 2014;14:869-82. [Crossref] [PubMed]
Cite this article as: Santoni SM, Winston T, Hoang P, Ma Z. Microsystems for electromechanical stimulations to engineered cardiac tissues. Microphysiol Syst 2018;2:11.


