
Prospects and challenges in engineering functional respiratory epithelium for in vitro and in vivo applications
Respiratory epithelium: prerequisites
Respiratory diseases such as asthma, chronic obstructive pulmonary disease, occupational lung diseases and pulmonary hypertension are the third most common cause of mortality worldwide affecting millions of people every year (1,2). Moreover, the number of patients suffering from chronic respiratory diseases has been increasing significantly due to allergies, infections and pollution (3). The effective treatment modalities for respiratory diseases are limited and thus, more efficient pharmacological approaches or bioengineering inspired solutions are required to meet clinical needs. Moreover, there is an urgent need for in vitro disease models with high microphysiological fidelity to better understand these diseases. Thus, for both therapeutic and research requirements, engineering of a functional respiratory epithelium is of high interest.
The respiratory epithelium covers the respiratory tract and is involved in several protective functions both as a physical barrier and a component of the innate immune system. It is a dynamic tissue that ordinarily undergoes slow and constant renewal process in health state; however extensive damage, lesions or other pathologies may occur in the architecture of the airway walls in patients with respiratory disorders (4). It also acts as first line of immune defense to guide the immune system during lung infection or injury. The mucociliary clearance in upper airway, and reduction of surface tension at distal airway further support the immune system to maintain the sterility (5).
The epithelial tissue maintains its integrity by undergoing dedifferentiation, proliferation, and redifferentiation stages to recover from physical or chemical injuries however sustained insults (e.g., chronic inflammation) could result in tissue remodelling and loss of function. The epithelial layer has the capability to modulate the tissue repair processes through the secretion of extracellular matrix (ECM) proteins and the interaction with ECM secreting interstitial fibroblasts. Upon injury, the fibroblasts layer underneath the basement membrane plays a critical role in supporting the epithelial repair. The synergistic activity of respiratory epithelium and underlying fibroblasts results in the formation of a provisional layer of ECM followed by the secretion of several growth factors, pro-inflammatory cytokines and chemokines (6-8). This provisional ECM supports the migration of epithelial layer to reconstitute the surface layer (8). The crosstalk between epithelial cells and fibroblasts via induction and release of cytokines is a complex process which further regulates remodelling and repair of epithelial tissue (9).
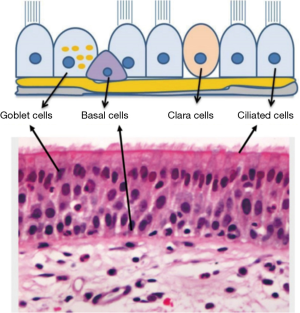
Simulating the regeneration of airway epithelium in vitro is a major challenge due to its complex organisation (10). The airway epithelium is composed of several types of cells; ciliated epithelial cells are predominant population in addition to secretory goblet and basal cells. Basal cells have progenitor potential for all other cell types in the airway. They have proliferation capacity and ability to differentiate to goblet and ciliated cells during the epithelium renewal (11). Goblet cells are of secretory nature producing complex glycoproteins known as mucins (mucin5A/C and mucin5B); the key element of particle and pathogen neutralisation machinery in the airways. They also facilitate the neutralisation of the encapsulated foreign particles to enable their removal by the ciliary cells (10). Therefore, the presence of ciliary cells, goblet cells as well as a layer of basal cells is essential for the development of functional respiratory epithelium (Figure 1) (12). Clearly the presence of multiple and functionally distinct cell types with different growth and differentiation patterns make the bioengineering of respiratory epithelium a complex task that requires close collaboration between cell biologists, materials scientists and tissue engineers.
Cell sources for engineering respiratory epithelium
The selection of right cell types for bioengineering of respiratory epithelium is an important challenge. Isolation of different cell types [i.e., basal cells, respiratory epithelial cells (RECs) and endothelial cells] from lung epithelium tissue samples is one possibility. The basal as well as suprabasal cells both have progenitor efficacy in embryonic stage and are capable of reconstituting a fully functional airway epithelium (13). However, suprabasal cells are not able to differentiate into organised epithelium in adult human and thus basal cells are the only known source of epithelial progenitors (14). These endogenous progenitor cells play an important role in lung regeneration, particularly in the early repair of the alveolar surface after injury and this regeneration capacity declines with the age. Given the limited availability and differentiation capacity of basal cells finding an alternative source of epithelium progenitor cells is a crucial step for bioengineering of respiratory epithelium. In this context, other epithelial cell sources e.g., nasal epithelium, buccal epithelium and skin epithelium have been reported as an epithelium substitute (Figure 2; Table 1). However, the buccal epithelium and skin epithelium could not gain much attention due to several limitations e.g., chances of hair growth, mucus stagnation and desquamation (in keratinocytes) (10), longer time required for local adaptation (buccal epithelium) (28,29) and also chances of dedifferentiation. However, there is still great potential for the success of epithelium regeneration via non-orthogonal grafts as shown by buccal, skin and bowel epithelium grafting for urinary tract rehabilitation (30). Potentially, the stem cell delivery can fulfil these demands due to their proliferation capacity and differentiation ability to adopt guided cell lineage/phenotype. Stem cells are already reported as a vital source for the epithelial differentiation ability and adopting the epithelium lineage after in vivo administration. Although there is a rapid development in embryonic stem cells (ESCs) and induced pluripotent stem cells (iPSCs) based research for in vitro differentiation towards the lung epithelium.
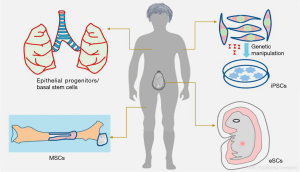
Table 1
| Cell types | Advantages | Limitations | References |
|---|---|---|---|
| Basal stem cells | Present within the tissue, active after injury, have good regeneration potential, native cells | Slow proliferation reported | (14-16) |
| MSCs | Have enormous proliferation capacity, non/low immunogenicity, autologous as well as allogenic cells can be used, safe source | Limited in vitro differentiation to the respiratory epithelium | (17,18) |
| iPSCs | Have enormous proliferation capacity, pluripotency can be induced, use of autologous cells is possible | Endodermal push is essential, requires pre-treatment (induction of pluripotency) | (19-21) |
| ESCs | Have enormous proliferation capacity, pluripotent in nature | Ethical issues, less safe to use, teratogenic potential | (21,22) |
| Lung fibroblasts/amniotic membrane | Part of tissue, provide the mesenchymal signal to the environment to induce the tissue repair | Only synergistic role, cannot be differentiated to respiratory epithelial cells on its own | (23-25) |
| Nasal epithelium | Enormous proliferation capacity, exhibit the stem cell properties in addition to the epithelium phenotype | Asymmetric growth rate which decline after cell passaging in vitro, less mucin production | (26,27) |
MSC, mesenchymal stem cell; iPSC, induced pluripotent stem cell; ESC, embryonic stem cell.
Epithelial basal/stem cells
Several lung specific cells such as multipotent alveolar cells, basal cells, basal airway stem cells, airway progenitors, alveolar progenitors and non-progenitor lineage have been discovered within the respiratory tissues (31). These endogenous cells bear an extensive ability to respond to tissue injury and regeneration of lost or damaged cells. The primary role of basal stem cells is epithelial homeostasis and the repair of any defect in the airway wall (32). Basal cells are a multipotent stem cell population of the pseudostratified airway epithelium. They are present in vicinity of the underlying basal lamina layer (hence are known as basal cells) and are primarily responsible for airway epithelial repair, regeneration, and remodelling process (16). Basal cells act as the stem cells for the trachea and airway epithelium and generate the differentiated cells during postnatal growth and maintain the adult airway epithelium during steady state as well as epithelial repair (15). The use of autologous epithelial stem cells and chondrocytes have shown potential regenerative capacity to replace the main bronchus once transplanted using decellularised trachea. The successful transplantation of such engineered tissue raises the hope for successful treatment of patients with serious clinical disorders such as bronchomalacia (33).
Basal cells have also been shown to support the epithelial differentiation of other cells e.g., stem cells in co-culture systems (34,35). The primary airway epithelium in in vitro cultures grown at the air-liquid interface (ALI) most closely resembles the phenotype of in vivo airway epithelia as demonstrated by the transcriptional profile (36). One of the key studies showing the effectiveness of airway epithelial cells (AECs) in inducing the MSCs differentiation into the epithelial phenotype was performed by Wang et al. Herein, the BM-MSCs and AECs were grown at ALI on semi-permeable filter membranes and around 10% MCSs transformed to epithelial phenotype, as evidenced by the presence of cytokeratin 18 and occludin (35).
Being a subpopulation of multipotent stem cells, basal cells have the ability to differentiate to multiple epithelium cell types in vitro (37), however, the regeneration capacity of adult respiratory epithelium is limited due to slow differentiation capacity. Further, they constitute a small portion of the total RECs and thus their isolation particularly in patients suffering from defects is a tedious process (38).
MSCs
MSCs are the most widely used cell source in cell therapy and regenerative medicine and they are also a potential source for the respiratory epithelium repair and regeneration. MSCs bear a number of favourable properties that make them suitable candidates for cell based therapy, i.e., easy extraction and the possibility to readily expand in culture without loss of multi-lineage capacity. Further, they are easy to transfect, allowing for simple ex vivo modification. The fact that bone marrow derived mesenchymal stem cells (BM-MSCs) are able to migrate to site specific areas in a range of diseased sites after implantation highlights their vital role as an ideal vector for therapeutic delivery. These cells already have proved their curative role in injured lung without any significant adverse immune responses. They even can support the tissue growth by modulating the immune response, e.g., through suppression of pro-Th2 cytokine IL-25 and reduction in number of activated and antigen-acquiring CD11c(+)CD11b(+) dendritic cells which protect the epithelium against Th2-mediated allergic airway inflammation and also airway hyper responsiveness (39). Finally, they ensure relatively non-immunogenic characteristics due to the absence of major histocompatibility complex II (MHCII) and co-stimulatory molecules (e.g., CD80, CD86 and CD40) as well as production of immune modulatory cytokines such as IL-10. These properties ensure the delivery of MSCs with minimum need for further immunomodulation or subsequent immunosuppressive therapy for the recipient (40).
MSCs also protect the lung from external injury and fibrosis by suppressing inflammatory cytokines (IFN-γ, IL-2, IL-1β, and IL-4) and production of reparative growth factors (circulating G-CSF and GM-CSF)(40,41). Previous studies suggested that the intravenous administration of LacZ-labeled MSCs cells into wild-type recipient mice after bleomycin-induced lung injury show that injected cells engrafted in recipient lung parenchyma with the morphological and molecular phenotype of type I pneumocytes to help in lung repair process (42). The autologous, labelled MSCs support the tracheal regeneration after aortic allografting (43). It is reported that human MSCs isolated from adult bone marrow can be differentiated into epithelial-like cells in the presence of following growth factors; keratinocyte growth factor, epidermal growth factor, hepatocytes growth factor and insulin growth factor 2 (complete differentiation) (17) or retinoic acid (partial differentiation) (18). Thus, MSCs-epithelial differentiation is based on signalling guidance, however the culture substrate also can influence the differentiation process (44). The co-culture of MSCs with AECs (34,35) or MRC-5 cells (43) is very efficient in adopting the epithelial lineage as they adopt the epithelial morphology and start expressing various epithelium markers e.g., cytokeratin 18, 19 and adherens junctions; E-cadherin and β-catenin.
BM-MSCs culture in the presence of modified small airway growth medium (SAGM) can result in differentiation into human type II alveolar epithelial cells in 15 days where differentiated cells express surfactant protein C (SPC), a specific functional epithelium marker (45). In vivo delivery of cultured BM-MSCs in various animal models resulted in the airway localization and adoption of an epithelial-like phenotype (46) and supporting tissue regeneration e.g., tracheal regeneration after aortic allografting (43) or Bleomycin induced lung injuries (42,47).
The experimental models suggest that another adult stem cells population; adipose-derived stem cells (ASCs) are also involved in epithelium maturation as they stimulate epithelial proliferation to make a multi-layered thick epithelium which results in epithelial covering on the transplanted graft (25). Fat derived MSCs were cultured on a collagen scaffold and the developed bioengineered tissue was transplanted to the tracheal defects in rats, which resulted in well differentiated airway epithelium and also showed the presence of neo-vascularised tissue within two weeks of transplantation (48). Another study suggests trans-tracheal delivery of short-term cultured MSCs results in enhanced airway localization and adoption of an epithelial-like phenotype (46). Using an in vitro scratch wound repair model, it was shown that the MSCs conditioned media facilitates wound repair in human type II alveolar epithelial cell line A549 cells (AECs) and also primary human small airway epithelial cells (SAECs) (49).
ESCs
ESCs are derived from the inner cell mass of a blastocyst (an early-stage preimplantation embryo). These cells have vital potential in developmental biology, medicine and pharmacology due to the ability to generate a diverse number of cell types and their pluripotent nature. Nevertheless, the application of ESCs in regenerative medicine is still limited due to the ethical requirements/ complexities and scientific considerations. The potential application of ESCs is discussed in a comprehensive review article by Varanou et al., 2008 (50). Most of the efforts using ESCs in respiratory regeneration are largely focused on obtaining distal lung epithelial phenotypes; the alveolar epithelium. Several methods of ESCs differentiation to the alveolar epithelium include recapitulating developmental signalling events, mimicking the physical environment, or forcibly reprogramming the ESCs nucleus (51,52). Murine ESCs have shown the ability to generate a fully differentiated functional tracheobronchial epithelium after being cultured at the air—liquid interface on acellular type I collagen—coated porous membranes (25).
ESCs can also be guided for the controlled differentiation to the respiratory epithelium present at upper airway by mimicking the endoderm developmental pathways using growth factor supplemented culture medium followed by ALI culture (53). Such a culture system resulted in maturation of tight junction-coupled, differentiated AECs with active cystic fibrosis transmembrane conductance regulator (CFTR) transport function. CFTR regulates the chloride and water transport across the epithelial layer. The culture environment was changed by addition of different growth factors [fibroblast growth factors 7 (FGF7), fibroblast growth factors 10 (FGF10), bone morphogenetic protein 4 (BMP4) and fibroblast growth factors 18 (FGF18)] at various time points to promote airway lineage development (21).
iPSCs
iPSCs are another type of pluripotent stem cells used for the respiratory epithelial differentiation (54) and to induce the repair process (55). The iPSCs are abundant stem cell source which are also amenable to gene editing and clonal expansion. These cells are produced by the reprogramming of somatic cells towards an embryonic state and can be easily grown from the patient’s own cells (56). The selective induction of iPSCs using the lung embryonic development pathway, results in production of at least 6 sub-types of epithelial cells; basal, goblet, Clara, ciliated, type I and type II alveolar epithelial cells (20). The endodermal push of iPSCs using the activin A and anterior foregut endoderm push by neutralising the TGF-β and BMP signalling are crucial steps to induce the endodermal lung regeneration using the iPSCs (19-21).
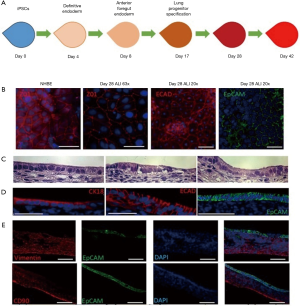
One study demonstrated that the generation of fully mature, multiciliated respiratory epithelium is possible using stepwise differentiation of iPSCs. In this study, the authors used the endoderm inducing medium for early growth of iPSCs and further adjusted the medium to facilitate transformation to motile multiciliated cells (MCCs) and finally grew the cells at air liquid interface in the presence of epithelium differentiation medium (19). These cells showed several characteristic features of native epithelium including pseudostratified polarized layer of forkhead box protein A2+ and NK2 homeobox 1 positive endodermal-derived epithelial cells. Interestingly, these iPSCs differentiated epithelium also included Clara cells with Clara cell 10 kD-positive vesicles, mucin 5A/C-positive goblet cells, multiciliated cells, and isolated basal cells (Figure 3). This study is a significant advance towards the development of fully functional epithelium and could enable modelling of a number of human respiratory diseases in vitro in the future (19). It is also demonstrated that human iPSCs can differentiate to type II pneumocytes (ATII cells) that attach and proliferate on decellularized lung matrices while simultaneously maintaining the epithelial lineage (21,54,57).
Miscellaneous cell sources (amniotic fluid stem/progenitor cells/lung fibroblasts)
Amniotic membrane as well as the amniotic fluid are the potential sources of stem cells which are easily accessible, non-tumorigenic and also capable of differentiating into a variety of cell lineages for tissue engineering and diagnostic applications such as cell karyotyping and other genetic and molecular tests (58). The stem cells derived from amniotic fluid are multipotent in nature and can be differentiated to all three-germ layer originated tissues e.g., bone, heart, liver, neuron and endothelium (59). Recently, human amniotic fluid stem cells (hAFSC) have been shown to integrate in lung epithelium with thyroid transcription factor 1 (TTF1) expression after microinjection into cultured mouse embryonic lung tissue. They also show transformation capacity into specific alveolar versus bronchiolar epithelial cell lineage due to their plasticity and ability to respond to different types of lung damage in different ways (60).
The tracheal epithelium was reconstructed in vitro when tracheal epithelial cells and fibroblasts were co-cultured on epithelial side and serosal side respectively to mimic the basement membrane like structure. This method resulted in pseudostratified columnar ciliated epithelium in addition to the goblet-like and basal cells with similar ratio of each type of differentiated cells identical to the native tissue (23). Other cell sources such as lung fibroblasts or lung endothelium are also able to modulate the in vitro regeneration capacity of airway epithelium due to provision of the tissue specific signalling. The use of fibroblasts conditioned medium in epithelial cell culture achieved well defined epithelium, which confirms the essential role of soluble factors in growth and differentiation of epithelial cells during epithelial–mesenchymal interaction (23-25,61). Müller et al., 2012 used nasal turbinate epithelium in in vitro culture as an alternative source for respiratory epithelium development with a respiratory-type morphology and normal ciliary activity. They cultured nasal turbinate epithelial cells at air liquid interface in the form of fully differentiated human AECs. This type of organotypic in vitro model can be useful to study the effect of viral particles, pollutants, allergens, or gases (62). Nasal epithelial cell and fibroblasts have also been reported to support the development of autologous tracheal graft to favour the natural regenesis of the tracheal epithelium with minimal fibrosis induction (26). The immune cells particularly the macrophages also reported to induce the in vitro proliferation of bovine bronchial epithelial cells (63,64).
There has been considerable progress in regenerative medicine approaches for the development of various tissues. However, the development of complex tissues such as respiratory epithelium is not straightforward and identifying the correct cell source is a major obstacle for the successful clinical application of such tissues. The presence of multiple cell types and their specific physiological functioning are key necessities for the success of the developed tissues. Patient specific basal stem cells have been proposed as the potential cell source for the development of respiratory tissue however these cells lose the differentiation and proliferation ability in time which is a major limiting factor for their application. Thus, there is a need for alternative sources of basal stem cells which are capable of differentiation to organised epithelium tissue. Based on the existing literature it is predicted that iPSCs could play a major role in the future of stem cell therapy for the controlled respiratory epithelium development due to their indefinite propagation ability and the fact that they can be maintained in culture for longer time without losing the pluripotency.
Use of biomaterials in respiratory epithelium engineering
Biomaterials play a significant role in tissue engineering applications and the selection of the suitable biomaterials in terms of both chemical and physical properties can provide the optimal niche for developing tissue engineered respiratory epithelium. This also holds true for in vitro engineering of a microphysiologically relevant tissue models via inclusion of pertinent ECM components. An ideal biomaterial based respiratory epithelium substrate should be nontoxic, biocompatible, biodegradable (if considered for in vivo applications) and strong enough to provide adequate cell support. Simultaneously it should be able to promote cell adhesion and should emulate the native tissue geometry to provide a biophysical microenvironment supporting the cell growth, migration and basal cell differentiation (35,65). Biomaterials can be selected for their ability to support the MSCs mediated growth factor performance, immunomodulation and also guiding/supporting controlled cell differentiation (66,67). The tissue engineering of respiratory epithelium is based on three basic approaches: (I) in situ homing in which scaffold materials provide the adhesion, proliferation and migratory support to the cells; (II) the cell sheet technology that uses the reversible thermosensitive property of N-isopropyl acrylamide based polymers to detach the intact cell sheets with their deposited ECM; and (III) air liquid interface technique which is widely used to promote the epithelium differentiation to a multi-lineage phenotype (10).
Thus, careful selection of biomaterials which can coordinate the differentiated cells into a functional assembly of respiratory tissue is essential (68). The successful in vitro development of respiratory epithelium models can facilitate our knowledge of complex cellular and molecular processes in healthy individuals and also in diseased patients. Using this information, optimal culture conditions/culture microenvironment can be identified to enhance the proliferative/cell phenotype maintenance capacity of the cells bearing differentiated characteristics of healthy pulmonary epithelium. Particularly, fabrication of biologically active scaffolds and matrices is an important consideration in developing bioengineered models of the respiratory tissue. For example, the matrices used for epithelial tissue development should maintain the controlled growth factor release for proliferation and differentiation in addition to the superior adhesion and migration of the cells. All in all, for respiratory epithelium development optimal conditions are essentially based on defining the culture medium, providing the native ECM microenvironment and also using the ALI for ciliary differentiation (69).
Natural biomaterials
Several natural biomaterials have been used for tissue engineering applications. Among them, collagen and hyaluronan are two most commonly used natural biomaterials for the ciliary differentiation of human RECs. However, in one study Ziegelaar et al., observed that a hyaluronic acid derivative scaffold (Hyaff®, Fidia, Italy) did not support the cell adhesion while collagen scaffolds (BiofleeceTM, Baxter, USA) encouraged cell spreading and adhesion. The presence of ciliated epithelium cells was confirmed using Ulex Europaeus agglutinin (UEA), lectin staining and SEM (scanning electron microscopy) analysis of cultured epithelium on the collagen scaffold (70). A recent study suggests that, bilayered collagen-hyaluronate scaffold facilitate the calu-3 cell differentiation, with enhanced mucin expression, increased ciliation and the formation of intercellular tight junctions (Figure 4) (71).
Other studies have shown that esterified hyaluronic acid (HYAFF®) promotes the ciliary differentiation of retinoic acid induced human RECs, however the mechanism remains unknown. It is expected that the presence of a hyaluronan-binding domain, CD44 and increase in hyaluronan-mediated motility receptors of RECs stimulate the epithelium differentiation on HYAFF® as compared to collagen based scaffolds (72,73). Hyaluronan further protects the clearance of tissue kallikrein (TK) and airway lactoperoxidase for integral epithelium functioning (74), thus could play a vital role in developing respiratory epithelium. Collagen gels also have been used for the differentiation of BM-MSCs to the epithelium lineage in a depth dependent seeding. The retinoic acid supplemented medium induces the MSCs to epithelial lineage differentiation (>80% cytokeratin-18 positive cells) if cultured on thick collagen gel (~2 mm) due to non-recognition of hard tissue culture surface to MSCs as compared to the thin scaffolds (44).
Fibrin gels have also demonstrated adequate potential for the growth and differentiation of RECs similar to the collagen coated surfaces. One of the common rationales for using fibrin is that this biomaterial can be developed from autologous sources (75). It has also been revealed that fibrin can be used as surgical adhesive and tissue constructs remained viable after 5 days in culture, when fibrin was used to fuse the chondrocytes and epithelium layer to develop in vitro trachea (76). Fibrin gels also support the mucociliary epithelium differentiation of RECs when embedded in a 3-dimensional fibrin gel co-cultured with tracheal fibroblasts (77).
Chitosan-gelatin hybrid hydrogels were also tested for REC culture which resulted in decent cell attachment, epithelial morphology, and growth with the presence of beating cilia. No cytotoxic effect or any significant induction of immune response was observed on J774 macrophages. Even the presence of mucins 2, mucin 5 and cytokeratin 13, markers for secretory goblet and squamous cells, showed the multilineage phenotype epithelium regeneration ability, thus Chitosan–gelatin biomaterials could provide another potential alternative for coating the tracheal prostheses to induce the epithelium regeneration/maintenance (78).
Using optimal scaffolds that can facilitate complex interactions between different cell types would be an essential part of engineering physiologically relevant airway models. In this context, a co-culture system can provide more functionality in an in vitro developed tissue due to close structural alignment of several types of cells arranged in 3D. Such co-culture systems can use the combination of epithelium, fibroblasts, stem cells, and/or immune cells for the development of differentiated epithelium. A co-culture system consisting of tracheal epithelium and gingival fibroblasts (GFBs) or ASCs confirmed the higher epithelial cell differentiation for reconstructing a pseudostratified epithelium on collagen scaffolds. The collagen scaffolds implanted into rat tracheal defects indicates the synergistic effects of GFBs and ASCs on tracheal epithelial regeneration (25).
Despite their favourable bio-instructive properties collagen, gelatin, fibrin or similar ECM materials are not widely considered for upper airway epithelium tissue engineering mainly due to their high-water content which makes the materials viscoelastic. The handling of hydrogel based materials is also more difficult due to their weak mechanical characteristics (79). However, the combined use of ECM based hydrogel materials with synthetic polymers can support the further use of such materials in upper respiratory tissue engineering (80,81).
Semisynthetic/synthetic biomaterials
To minimise the limitations associated with soft material based hydrogels particularly the mechanical properties, application of synthetic materials has been suggested either on their own or in combination with ECM derived matrices. For example, respiratory epithelium cells seeded on tubular prostheses made up of porous polyurethane or expanded polytetrafluorethylene have shown potential for airway reconstruction in animal model. This technique supported the epithelialization on the luminal surfaces and vigorous squamous epithelium cell layers in the form of single and (predominantly) multiple layers, however differentiated ciliated or mucous cells were not identified (82).
The main advantages of using synthetic scaffold materials is their robustness and ability to control the mechanical properties of the developed tissue. However, synthetic materials could also be difficult to process into a highly porous structure and sometime mechanically brittle. Thus, ideal scaffold materials may be composed of combination of natural and synthetic materials having sufficient mechanical stability and supporting the bio-instructive environment for optimal cell growth and proliferation.
In another study a polypropylene and collagen based scaffold pre-clotted with peripheral blood was implanted to the circular defect in trachea in adult beagles. This scaffold demonstrated sufficient mechanical strength for tissue regeneration. The ciliated epithelium regenerated within one month of surgery and the scaffolds were fully covered with regenerated mucosa. The presence of newly formed cartilaginous tissue was also detected in the specimens after 8–12 months of implantation (83). Other examples include tracheal prosthesis composed of gelatin coated proline mesh and polypropylene rings that are seeded with autogenous oral mucosa which have been shown to promote the intrathoracic trachea reconstruction within six months of transplantation (29).
The use of Pluronic F-127 hydrogel facilitated the in vivo regeneration of lung tissue while polyglycolic acid resulted in foreign body response that altered the integrity of the developing lung tissue, however both polymers were found excellent for culture of somatic lung progenitor cells types in vitro (84). Furthermore, collagen and alginate coated PLLA/titanium implant material have also shown superior proliferation and migration of human RECs compared to PLLA film surface alone (85).
Titanium based scaffolds
Titanium is one of the most widely used materials in tracheal replacement due to its mechanical properties that ensures the integrity of patients’ upper airway by preventing airway collapse. Importantly titanium supports the growth and proliferation of respiratory epithelium both in vitro (86) and in vivo (26,87). A titanium based hybrid system composed of a macroporous titanium structure filled with a microporous biodegradable poly (L-lactic acid) (PLLA) polymer was shown to support human primary respiratory epithelium growth. In addition, it was able to guide PKH26 labelled fibroblasts migration due to built-in porosity gradients in spatial and temporal manner (86). This approach can help to counter the fibroblast mediated stenosis in the implants. Further, layer by layer coating of collagen and alginate on these PLLA/titanium implants helped in epithelialization of implant with respect to the amount of tissue integration (85). Recently, surgically induced partial segmental tracheal defects were treated using the titanium scaffold based hybrid bi-layered tracheal construct bearing autologous nasal RECs and seeded fibroblasts (87). Overall, titanium is a highly desirable biomaterial for respiratory tissue replacement with potential to support the regeneration of the epithelial component. The data suggest that titanium based materials will have significant clinical application in tracheal implant for the prevention of excessive fibroblast migration and proliferation induced restenosis which is a major complication in many tracheal implants.
Other approaches (cell sheet technology/decellularised tissue based scaffolds)
Decellularised tissue based scaffolds, which are composed of native ECM, are typically derived by processes that involves removal of cellular component from tissues or organs that can preserve the complex composition and three-dimensional (3D) ultrastructure of the native tissue. The use of decellularised whole lungs by perfusion of detergents could be used to prepare 3D scaffolds composed of acellular vasculature, airways and alveoli and is a viable strategy for lung regeneration (88,89). The seeded epithelium and endothelium displayed remarkable hierarchical organization within the decellularised matrix. This type of decellularised scaffolds are comprised solely of lung ECM with appropriate 3D architecture and region-specific cues for cellular adhesion. The subsequent developed lungs can be ventilated and perfused with blood and their function, including gas exchange, has been shown to be partially intact (90,91).
Other potential tissue engineering approaches such as cell sheet technology can further maximise the re-epithelialisation of the tracheobronchial airway if used in combination with biomaterials without inducing potential inflammatory effects following implantation (92,93). The remedial action of epithelial cell sheet on a pre-vascularised Dacron® PET graft could accelerate the development of mature pseudostratified columnar epithelium in a rabbit trachea within four weeks which was not shown in the absence of epithelial cell sheet (92). This suggests that the combination of cell sheet technology with other biomaterial constructs based multifactorial approach could maximise re-epithelialisation of the tracheobronchial airway, and can facilitate the clinical application of developed tissue construct (94).
Lung transplantation is a potential solution for replacing the diseased lung tissue however this approach suffers from major limitations such as shortage of donor tissue, immunological rejection and intensive immunosuppression required. Bioengineering of functional lung tissue could clearly offer an alternative to lung transplantation. While different approaches including scaffold free cell sheet technique and several scaffold materials (from natural to synthetic) have been used for the development of respiratory epithelium none could achieve the optimum functionality compared to the use of decellulrised tissue scaffolds. This is due to the fact that decellularised tissue scaffolds maintain the complex composition and three-dimensional ultrastructure of the ECM. Given the limited availability of decellularised tissue future research should focus on further optimisation of bioinspired materials that mimic the composition, structure and mechanical properties of the native ECM. Another area of development could be in titanium based implant materials for various laryngological applications due to titanium’s mechanical properties in addition to its excellent biocompatibility.
Immune cells in respiratory epithelium remodelling and regeneration
The consideration of immune response is an essential element in bioengineering of any implants made of foreign biomaterials or allogenic/xenogeneic cells and components. The acceptance of autologous cells by human body is well known and it does not need any immune suppression. For example, bioengineered trachea developed using autologous RECs and chondrocytes has been successfully tested in patients (33). However, the implantation of allogenic cells or biomaterials can lead to adverse immune responses or even rejection due to activation of different innate and adaptive immune cells. Thus, it remains an important challenge to overcome the immunological barriers for successful implementation of any developed tissue construct. Newer strategies are needed to control these immune responses e.g., use of smart biomaterials or induction of antigen-specific peripheral tolerance or suppression of autoreactive immune cells. This requires a fine coordination among the cell biologist, immunologist and biomaterials scientist for innovative solutions for such tissue regeneration approaches (95).
In addition, the immune system has been shown to play a vital role in the epithelial repair, regeneration, and remodelling after injury or in many tissues including respiratory epithelium (5,96,97). Furthermore, there is a close cross-talk between different components of the immune system and respiratory epithelium in organising innate immune responses against infection. For example, in addition to providing a physical barrier against pathogens the respiratory epithelium is also equipped with a host of anti-bacterial defense mechanisms such as mucociliary function and produces soluble factors such as defensins that play a key role in innate immune responses against pathogens. The wound healing in epithelium tissue is regulated by various immune cells such as neutrophils, monocytes/macrophages, lymphocytes, and mast cells. These immune cells are activated once the injured tissue produces chemokines to modulate the complex repair processes (98). Particularly, the supportive role of monocytes, macrophages in epithelium remodelling/regeneration is reported widely in literature. This is particularly relevant to developing in vitro models of lung epithelium. The co-culture of HCl-induced human lung epithelial cells with monocytes promote the epithelial-mesenchymal transition; enhanced production of interleukin-8, platelets derived growth factor and increased collagen deposition and lower epithelial markers (99). Macrophages play a vital role in wound healing of epithelium tissue by producing several growth factors and bioactive substances such as epidermal growth factor to promote the cellular proliferation and functional tissue regeneration (100). Alveolar macrophages and neutrophils promote alveologenesis during development and also protect the epithelium by clearing proteinaceous debris, neutralising the pathogens that escape the mucociliary defense mechanism, and modulating the inflammatory milieu (101,102). Infiltrating leukocytes support the epithelium repair during the proliferation stage by secreting several factors similar to the resident cells (103). The role of leucocytes in lung development was also proved in CD18-deficient mice that show impaired leukocyte trafficking and impaired lung generation (104). It was shown that the macrophages also contribute in tissue repair following injury by releasing growth factors into the resident milieu. Back in 1990, it was demonstrated that culturing the pulmonary epithelium in the presence of macrophage-conditioned medium showed a significantly better cell growth. Moreover, the co-culture of macrophages and bronchial epithelial cells led to the increased cell numbers with keratin positive staining (63). However, these findings have not been yet valorized for facilitation of in vitro respiratory epithelium regeneration. Further extensive studies are required to validate the role of immune system in respiratory epithelium remodelling and regeneration.
In a similar way, respiratory epithelium potentially also regulates the airway inflammation by regulating the function of the immune cells (105-107). Epithelial cells support the immune response by acting as a barrier against physical or chemical exposure and producing a range of molecules e.g., antimicrobial compounds and range of receptors, including the Toll-like receptors and nucleotide oligomerization domain-like receptors, to produce the pro-inflammatory molecules (100,108,109). These pro-inflammatory molecules i.e., cytokines, chemokines and damage associated molecular patterns (DAMPs) communicate with the immune cells to maintain the sterility of lungs (5,97). The pathogen recognition via Toll-like receptors and RIG-I-like receptors results in secretion of specific defense molecules such as mucins, antimicrobial peptides (AMPs) and reactive oxygen species (ROS) (110). GM-CSF and M-CSF released by airway fibroblasts and epithelial cells support the survival and accumulation of monocyte/macrophages (111). The epithelium mediated monocytes-macrophage differentiation is also reported in literature as evidenced by production of colony-stimulating factor (CSFs) (112,113) from epithelium or without even induction by CSFs (114). Thus, for the development of more refined epithelial tissue models in future one should also include the immune component in their design.
It is clear that a functional respiratory epithelium in essential for delivering efficient innate immune protection in the respiratory tract. Functions such as mucociliary clearance in conducting airways, reduction of surface tension in the alveoli are crucial for maintaining respiratory tract homeostasis and prevention of infection. In addition, upon injury or infection the respiratory epithelium instructs different components of the immune system to orchestrate clearance of pathogens and organised healing of the injured tissue. Thus, understanding the influence of immune cells on in vitro epithelium development and the nature of cross-talks between immune and epithelial cells could guide the strategies to improve in vivo efficacy of cell-based constructs for future bioengineering of respiratory epithelium.
Respiratory epithelium models
A combination of conventional 2D cultures using primary cells or cell lines, animal models and, more recently, organotypic cultures are regularly used to study different aspects of airway epithelium pathophysiology. Each of these platforms offer some advantages and at the same time differ from a number of limitations (115). The designated tissue model should be simple, biologically relevant, reproducible and should be able to illustrate a close comparison to the native tissue in addition to the fidelity of tissue specific functionality.
Several in vitro culture models for respiratory epithelium have been developed over the years in monoculture or co-culture form. These models range from 2D submerged epithelial culture (116) to 3D air liquid interface models (117,118). Most of the 2D models consist of single layer of tracheobronchial epithelial immortalised cell lines cultured on a semi-permeable culture insert at an ALI. This process induces cell polarisation, ciliary differentiation and mucus production to make a fully differentiated respiratory epithelium. Several primary and immortalized epithelial cells have been used to develop these models. It is reported that the primary AECs grown under ALI closely recapitulate the transcriptional profile of in vivo airway epithelia (36). Cultured human AEC line (Calu-3) is one of the most widely used models investigated to understand the mechanism behind the various respiratory functions, tissue remodelling and inflammatory responses. It is a well-differentiated and well characterized cell line which is derived from human bronchial submucosal glands (119). Such models are useful as a tool for drug transport studies (120,121), however it is reported that they are not appropriate in toxicity studies due to the lack of physiological complexity of the airways (122,123) and thus, hindering the successful generation of new therapeutic agents. Considering the vital role of fibroblasts and immune cells including dendritic cells and macrophages, the construction of airway models using these cells in addition to the respiratory cells would provide more insight on their role in respiratory epithelium regeneration.
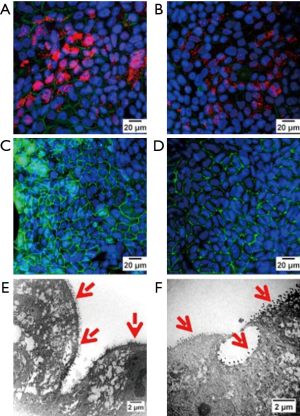
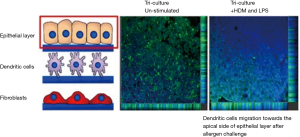
It is of particular interest to assess the influence of these cells in co-culture systems to understand the complex cellular processes. For example, it is demonstrated that the co-cultures are more responsive against combustion-derived nanoparticles (NPs) (DEP) and manufactured NPs [single-walled carbon nanotubes (SWCNTs) and titanium dioxide (TiO2)] to the monoculture as shown by co-culturing of A549 human epithelial lung cells, human monocyte-derived macrophages and monocyte-derived dendritic cells (MDDCs). The co-cultured cells showed a synergistic effect against the exposure of NPs (124). In contrast to co-culture, monoculture models showed minimal reaction to inflammatory stress, exhibited reduced oxidative stress, and increased pro-inflammatory responses (124). Our group recently used epithelial cells, dendritic cells and fibroblasts to make a 3D airway model using PET electro-spun fibres scaffolds which confirmed the increase in transepithelial electrical resistance (TEER) and also tight junction formation in epithelial cells-fibroblast co-cultures, thus the developed tissue substitute demonstrated a closer functional representation of native tissue (Figure 5). In this immunocompetent model, the immune cells also showed the migration potential in response to the allergen exposure (125). In another study, an organotypic in vitro culture model was developed using the epithelium cells harvested from nasal turbinates, which resulted in fully differentiated human airway epithelium (62).
3D spheroid culture of lung AECs on Matrigel® could provide a good alternative epithelial model for high-throughput screening of pharmaceutical agents (15). To understand the lentiviral transduction on airway epithelial reconstitution; 3D spheroid culture of airway epithelium from mature human fetal tracheas and airway xenografts were transduced using a HIV-1-derived VSV-G pseudotyped lentiviral vector. This study confirmed that 3D culture repopulated the denuded basement membrane to produce a well-differentiated airways epithelium (126). These 3D spheroid models can also be achieved without using the tissue culture inserts and are developed as lumen surrounded by epithelial cell layer and the apical surface of goblet and ciliated cells pointing towards the lumen (15,127). Some interesting in vitro respiratory models are also available commercially e.g., MucilAir™ and EpiAirway-FT®. MucilAir™ consists of epithelial cells cultured at an ALI representing a fully differentiated and functional respiratory epithelium (118). The EpiAirway-FT® model is based on culturing of epithelium on scaffold substrates embedded with co-cultured normal human stromal fibroblasts at air liquid interface. This model is also of great interest due to emulation native tissue structure, which can improve cell-cell signalling and functionality (117). By using 3D cell models like MucilAir™, EpiAirway-FT® and freshly isolated immune cells, robust co-culture systems could be developed for studying the inflammatory respiratory diseases like asthma or COPD, as these methods use the primary cells which are closer to the native tissue morphology.
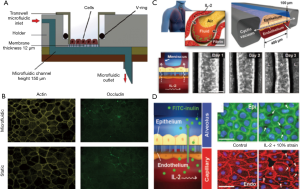
Another advanced technology in the development of in vitro tissue models is tissue on a chip models that can effectively emulate the native physiology, complex disease processes and also drug responses (128). These integrated models are made up of continuous perfused chambers occupied by the arranged cells to recapitulate the tissue- and organ-level physiology and typically allow real time imaging as well as the analysis of biochemical and metabolic readouts under physiologically relevant microenvironment (129). The microfluidic-based in vitro devices are made up of the micron-size channels with larger surface area to volume ratio similar to native tissue structure. Thus, these systems are becoming attractive platforms for disease modelling and testing new drug leads. A novel microfluidic model based on polydimethylsiloxane was fabricated using the soft lithography technique, to show the wound healing in cultured A549 epithelial cells and also the growth factor mediated wound repair after H2O2 or bleomycin induced injury (130). In a similar way, Blume et al. designed a dynamic 3D model in which fully differentiated primary bronchial epithelial cell loaded filter supports were inserted into the microfluidic culture system where nutrients were supplied from basolateral side. This type of model effectively facilitates the kinetic analysis of barrier responses against the changes in the local microenvironment (131) (Figure 6A,B). Recently, a silicone elastomer based biomimetic microfluidic device was prepared to mimic the pulmonary oedema. It recapitulates the alveolar capillary interface of lung in which human pulmonary epithelial and endothelial cells were seeded opposite to micro-channels in contact with air or liquid in case of endothelium. This type of micro devices can lead to the identification of new therapeutic moieties e.g., in this case, angiopoietin-1 (Ang-1) and a new transient receptor potential vanilloid 4 (TRPV4) ion channel inhibitor (GSK2193874) for oedema treatment (Figure 6C,D) (132).
The in vitro tissue models are widely used for screening and classification of chemicals, especially in testing drugs at the preclinical stages. The established cell lines (e.g., Calu 3) have been successfully employed in respiratory research particularity in drug transport studies. However, there are also in vitro culture models comprised of primary epithelium such as MucilAir™, EpiAirway™ which are 3D human airway epithelium model systems that can be used for long term toxicity testing, chemical risk assessment and drug delivery. In addition, such models can be developed using diseased cells to investigate various processes under diseased conditions. The authors believe with recent advances in cell preservation techniques, real-time monitoring of cellular responses and fabrication of microfluidic devices there is potential for development of more advanced and physiologically relevant in vitro respiratory models in the near future.
Future outlook
Different strategies aimed at development of physiologically relevant models of respiratory epithelium are gaining wide attention in regenerative medicine field, as well as in pharmaceutical industry which can lead to significant progress in the treatment of respiratory diseases. There is a need to identify means for faster differentiation of epithelial cells as well as more sophisticated but user-friendly 3D scaffolds and lab on chip systems that allow simulating in vivo like conditions and could enable real-time testing of barrier function and cellular responses. For this, it is of utmost importance to use the cell lineage of correct phenotype to guide the appropriate regeneration for the development of such complex tissue. In addition to the cell sources, the synchronisation of differentiated cells to make a functional tissue assembly is another equally important task. Several cell sources have been considered due to their potential regeneration capacity or differentiation ability towards the epithelial like cell lineage and their ability to be used for in vivo applications such as regeneration of extensive epithelial tissue losses. Such advances could pave the way for developing cell based therapies in various respiratory disorders where body’s natural regenerative capacities are not sufficient. The use of ALI culture technique, co-culture systems or application of biomaterials based culture matrices or spheres could lead to significant advances in the development of more physiologically representative tissue models for drug discovery and disease modelling. Additionally, the use of primary cells further allows more relevant studies such as incorporation of genotype specific patient cells, and would open new doors for highly beneficial personalized diagnostic and treatment options.
Acknowledgments
Funding: This work was supported by CASCADE-FELLOWS Scheme of EU Marie Curie COFUND in association to the University of Nottingham and EU FP7 IMMODGEL (602694) project awarded to AM Ghaemmaghami and NE Vrana under FP7-HEALTH-2013-INNOVATION-1 Programme.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.2103710.21037/mps.2017.09.01). Nihal E Vrana reports employment by Protip Medical and SPARTHA. PK and AG have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Alwan A. editor. Global Status Report on Noncommunicable Diseases 2010. Geneva, Switzerland: World Health Organization, 2010.
- Bousquet J, Khaltaev N. Global Surveillance, Prevention and Control of Chronic Respiratory Diseases: A Comprehensive Approach. Geneva, Switzerland: World Health Organization, 2007.
- Bousquet J, Dahl R, Khaltaev N. Global Alliance against Chronic Respiratory Diseases. Allergy 2007;62:216-23. [Crossref] [PubMed]
- Minoo P, King RJ. Epithelial-Mesenchymal Interactions in Lung Development. Annu Rev Physiol 1994;56:13-45. [Crossref] [PubMed]
- Whitsett JA, Alenghat T. Respiratory Epithelial Cells Orchestrate Pulmonary Innate Immunity. Nat Immunol 2015;16:27-35. [Crossref] [PubMed]
- Coraux C, Roux J, Jolly T, et al. Epithelial Cell–Extracellular Matrix Interactions and Stem Cells in Airway Epithelial Regeneration. Proc Am Thorac Soc 2008;5:689-94. [Crossref] [PubMed]
- Nishioka M, Venkatesan N, Dessalle K, et al. Fibroblast-Epithelial Cell Interactions Drive Epithelial-Mesenchymal Transition Differently in Cells from Normal and COPD Patients. Respir Res 2015;16:72. [Crossref] [PubMed]
- Sacco O, Silvestri M, Sabatini F, et al. Epithelial Cells and Fibroblasts: Structural Repair and Remodelling in the Airways. Paediatr Respir Rev 2004;5 Suppl A:S35-40.
- Knight D. Epithelium-Fibroblast Interactions in Response to Airway Inflammation. Immunol Cell Biol 2001;79:160-4. [Crossref] [PubMed]
- Vrana NE, Lavalle P, Dokmeci MR, et al. Engineering Functional Epithelium for Regenerative Medicine and In Vitro Organ Models: A Review. Tissue Eng Part B Rev 2013;19:529-43. [Crossref] [PubMed]
- Hong KU, Reynolds SD, Watkins S, et al. In Vivo Differentiation Potential of Tracheal Basal Cells: Evidence for Multipotent and Unipotent Subpopulations. Am J Physiol Lung Cell Mol Physiol 2004;286:L643-9. [Crossref] [PubMed]
- Hogan BL, Barkauskas CE, Chapman HA, et al. Repair and Regeneration of the Respiratory System: Complexity, Plasticity, and Mechanisms of Lung Stem Cell Function. Cell Stem Cell 2014;15:123-38. [Crossref] [PubMed]
- Avril-Delplanque A, Casal I, Castillon N, et al. Aquaporin-3 Expression in Human Fetal Airway Epithelial Progenitor Cells. Stem Cells 2005;23:992-1001. [Crossref] [PubMed]
- Hajj R, Baranek T, Le Naour R, et al. Basal Cells of the Human Adult Airway Surface Epithelium Retain Transit-Amplifying Cell Properties. Stem Cells 2007;25:139-48. [Crossref] [PubMed]
- Rock JR, Onaitis MW, Rawlins EL, et al. Basal Cells as Stem Cells of the Mouse Trachea and Human Airway Epithelium. Proc Natl Acad Sci U S A 2009;106:12771-5. [Crossref] [PubMed]
- Rock JR, Randell SH, Hogan BLM. Airway Basal Stem Cells: A Perspective on Their Roles in Epithelial Homeostasis and Remodeling. Dis Model Mech 2010;3:545-56. [Crossref] [PubMed]
- Păunescu V, Deak E, Herman D, et al. In Vitro Differentiation of Human Mesenchymal Stem Cells to Epithelial Lineage. J Cell Mol Med 2007;11:502-8. [Crossref] [PubMed]
- Ricciardi M, Malpeli G, Bifari F, et al. Comparison of Epithelial Differentiation and Immune Regulatory Properties of Mesenchymal Stromal Cells Derived from Human Lung and Bone Marrow. PLoS One 2012;7:e35639 [Crossref] [PubMed]
- Firth AL, Dargitz CT, Qualls SJ, et al. Generation of Multiciliated Cells in Functional Airway Epithelia from Human Induced Pluripotent Stem Cells. Proc Natl Acad Sci U S A 2014;111:E1723-30. [Crossref] [PubMed]
- Huang SXL, Islam MN, O'Neill J, et al. Efficient Generation of Lung and Airway Epithelial Cells from Human Pluripotent Stem Cells. Nat Biotechnol 2014;32:84-91. [Crossref] [PubMed]
- Wong AP, Bear CE, Chin S, et al. Directed Differentiation of Human Pluripotent Stem Cells Into Mature Airway Epithelia Expressing Functional CFTR Protein. Nat Biotechnol 2012;30:876-82. [Crossref] [PubMed]
- Coraux C, Nawrocki-Raby B, Hinnrasky J, et al. Embryonic Stem Cells Generate Airway Epithelial Tissue. Am J Respir Cell Mol Biol 2005;32:87-92. [Crossref] [PubMed]
- Goto Y, Noguchi Y, Nomura A, et al. In Vitro Reconstitution of the Tracheal Epithelium. Am J Respir Cell Mol Biol 1999;20:312-8. [Crossref] [PubMed]
- Kobayashi K, Nomoto Y, Suzuki T, et al. Effect of Fibroblasts on Tracheal Epithelial Regeneration In Vitro. Tissue Eng 2006;12:2619-28. [Crossref] [PubMed]
- Kobayashi K, Suzuki T, Nomoto Y, et al. A Tissue-engineered Trachea Derived from a Framed Collagen Scaffold, Gingival Fibroblasts and Adipose-derived Stem Cells. Biomaterials 2010;31:4855-63. [Crossref] [PubMed]
- Mohd Heikal MY, Aminuddin BS, Jeevanan J, et al. Autologous Implantation of Bilayered Tissue-Engineered Respiratory Epithelium for Tracheal Mucosal Regenesis in a Sheep Model. Cells Tissues Organs 2010;192:292-302. [Crossref] [PubMed]
- Noruddin NAA, Saim AB, Chua KH, et al. Human Nasal Turbinates as a Viable Source of Respiratory Epithelial Cells Using Co-Culture System Versus Dispase Dissociation Technique. Laryngoscope 2007;117:2139-45. [Crossref] [PubMed]
- Delaere P, Vranckx J, Verleden G, et al. Tracheal Allotransplantation after Withdrawal of Immunosuppressive Therapy. N Engl J Med 2010;362:138-45. [Crossref] [PubMed]
- Suh SW, Kim J, Baek CH, et al. Replacement of a Tracheal Defect with Autogenous Mucosa Lined Tracheal Prosthesis Made from Polypropylene Mesh. ASAIO J 2001;47:496-500. [Crossref] [PubMed]
- Li Y, Liu W, Hayward SW, et al. Plasticity of the Urothelial Phenotype: Effects of Gastro-Intestinal Mesenchyme/Stroma and Implications for Urinary Tract Reconstruction. Differentiation 2000;66:126-35. [Crossref] [PubMed]
- Kotton DN, Morrisey EE. Lung Regeneration: Mechanisms, Applications and Emerging Stem Cell Populations. Nat Med 2014;20:822-32. [Crossref] [PubMed]
- Giangreco A, Arwert EN, Rosewell IR, et al. Stem Cells are Dispensable for Lung Homeostasis but Restore Airways After Injury. Proc Natl Acad Sci U S A 2009;106:9286-91. [Crossref] [PubMed]
- Macchiarini P, Jungebluth P, Go T, et al. Clinical Transplantation of a Tissue-Engineered Airway. Lancet 2008;372:2023-30. [Crossref] [PubMed]
- Spees JL, Olson SD, Ylostalo J, et al. Differentiation, Cell Fusion, and Nuclear Fusion Dring Ex Vivo Repair of Epithelium by Human Ault Stem Cells from Bone Marrow Stroma. Proc Natl Acad Sci U S A 2003;100:2397-402. [Crossref] [PubMed]
- Wang G, Bunnell BA, Painter RG, et al. Adult Stem Cells from Bone Marrow Stroma Differentiate into Airway Epithelial Cells: Potential Therapy for Cystic Fibrosis. Proc Natl Acad Sci U S A 2005;102:186-91. [Crossref] [PubMed]
- Pezzulo AA, Starner TD, Scheetz TE, et al. The Air-Liquid Interface and Use of Primary Cell Cultures Are Important to Recapitulate the Transcriptional Profile of In Vivo Airway Epithelia. Am J Physiol Lung Cell Mol Physiol 2011;300:L25-L31. [Crossref] [PubMed]
- Rawlins EL, Hogan BL. Epithelial Stem Cells of the Lung: Privileged Few or Opportunities for Many? Development 2006;133:2455-65. [Crossref] [PubMed]
- Soleas JP, Paz A, Marcus P, et al. Engineering Airway Epithelium. J Biomed Biotechnol 2012;2012:982971.
- Duong KM, Arikkatt J, Ullah MA, et al. Immunomodulation of Airway Epithelium Cell Activation by Mesenchymal Stromal Cells Ameliorates House Dust Mite–Induced Airway Inflammation in Mice. Am J Respir Cell Mol Biol 2015;53:615-24. [Crossref] [PubMed]
- Sage EK, Loebinger MR, Polak J, et al. The Role of Bone Marrow-derived Stem Cells in Lung Regeneration and Repair. In: Bhatia S. editor. StemBook. The Stem Cell Res Community, 2008.
- Rojas M, Xu J, Woods CR, et al. Bone Marrow–Derived Mesenchymal Stem Cells in Repair of the Injured Lung. Am J Respir Cell Mol Biol 2005;33:145-52. [Crossref] [PubMed]
- Kotton DN, Ma BY, Cardoso WV, et al. Bone Marrow-Derived Cells as Progenitors of Lung Alveolar Epithelium. Development 2001;128:5181-8. [PubMed]
- Seguin A, Baccari S, Holder-Espinasse M, et al. Tracheal Regeneration: Evidence of Bone Marrow Mesenchymal Stem Cell Involvement. J Thorac Cardiovasc Surg 2013;145:1297-1304.e2. [Crossref] [PubMed]
- Takebayashi T, Horii T, Denno H, et al. Human Mesenchymal Stem Cells Differentiate to Epithelial Cells when Cultured on Thick Collagen Gel. Biomed Mater Eng 2013;23:143-53. [PubMed]
- Ma N, Gai H, Mei J, et al. Bone Marrow Mesenchymal Stem Cells Can Differentiate Into Type II Alveolar Epithelial Cells In Vitro. Cell Biol Int 2011;35:1261-6. [Crossref] [PubMed]
- Wong AP, Dutly AE, Sacher A, et al. Targeted Cell Replacement with Bone Marrow Cells for Airway Epithelial Regeneration. Am J Physiol Lung Cell Mol Physiol 2007;293:L740-52. [Crossref] [PubMed]
- Zhao F, Zhang YF, Liu YG, et al. Therapeutic Effects of Bone Marrow-Derived Mesenchymal Stem Cells Engraftment on Bleomycin-Induced Lung Injury in Rats. Transplant Proc 2008;40:1700-5. [Crossref] [PubMed]
- Suzuki T, Kobayashi K, Tada Y, et al. Regeneration of the Trachea Using a Bioengineered Scaffold with Adipose-Derived Stem Cells. Ann Otol Rhinol Laryngol 2008;117:453-63. [Crossref] [PubMed]
- Akram KM, Samad S, Spiteri MA, et al. Mesenchymal Stem Cells Promote Alveolar Epithelial Cell Wound Repair In Vitro Through Distinct Migratory and Paracrine Mechanisms. Respir Res 2013;14:9. [Crossref] [PubMed]
- Varanou A, Page CP, Minger SL. Human Embryonic Stem Cells and Lung Regeneration. Br J Pharmacol 2008;155:316-25. [Crossref] [PubMed]
- Rippon HJ, Lane S, Qin M, et al. Embryonic Stem Cells as a Source of Pulmonary Epithelium In Vitro and In Vivo. Proc Am Thorac Soc 2008;5:717-22. [Crossref] [PubMed]
- Samadikuchaksaraei A, Cohen S, Isaac K, et al. Derivation of Distal Airway Epithelium from Human Embryonic Stem Cells. Tissue Eng 2006;12:867-75. [Crossref] [PubMed]
- Mou H, Zhao R, Sherwood R, et al. Generation of Multipotent Lung and Airway Progenitors from Mouse ESCs and Patient-Specific Cystic Fibrosis iPSCs. Cell Stem Cell 2012;10:385-97. [Crossref] [PubMed]
- Ghaedi M, Calle EA, Mendez JJ, et al. Human iPS Cell–Derived Alveolar Epithelium Repopulates Lung Extracellular Matrix. J Clin Invest 2013;123:4950-62. [Crossref] [PubMed]
- Basma H, Gunji Y, Iwasawa S, et al. Reprogramming of COPD Lung Fibroblasts Through Formation of Induced Pluripotent Stem Cells. Am J Physiol Lung Cell Mol Physiol 2014;306:L552-65. [Crossref] [PubMed]
- Yu J, Vodyanik MA, Smuga-Otto K, et al. Induced Pluripotent Stem Cell Lines Derived from Human Somatic Cells. Science 2007;318:1917-20. [Crossref] [PubMed]
- Gilpin SE, Ren X, Okamoto T, et al. Enhanced Lung Epithelial Specification of Human Induced Pluripotent Stem Cells on Decellularized Lung Matrix. Ann Thorac Surg 2014;98:1721-9. [Crossref] [PubMed]
- Rennie K, Gruslin A, Hengstschläger M, et al. Applications of Amniotic Membrane and Fluid in Stem Cell Biology and Regenerative Medicine. Stem Cells Int 2012;2012:13.
- De Coppi P, Bartsch G Jr, Siddiqui MM, et al. Isolation of Amniotic Stem Cell Lines with Potential for Therapy. Nat Biotechnol 2007;25:100-6. [Crossref] [PubMed]
- Carraro G, Perin L, Sedrakyan S, et al. Human Amniotic Fluid Stem Cells Can Integrate and Differentiate into Epithelial Lung Lineages. Stem Cells 2008;26:2902-11. [Crossref] [PubMed]
- Okano W, Nomoto Y, Wada I, et al. Bioengineered Trachea with Fibroblasts in a Rabbit Model. Ann Otol Rhinol Laryngol 2009;118:796-804. [Crossref] [PubMed]
- Müller L, Brighton LE, Carson JL, et al. Culturing of Human Nasal Epithelial Cells at the Air Liquid Interface. J Vis Exp 2013;(80).
- Takizawa H, Beckmann JD, Shoji S, et al. Pulmonary Macrophages Can Stimulate Cell Growth of Bovine Bronchial Epithelial Cells. Am J Respir Cell Mol Biol 1990;2:245-55. [Crossref] [PubMed]
- Verspohl EJ, Podlogar J. LPS-Induced Proliferation and Chemokine Secretion from BEAS-2B Cells. Pharmacology & Pharmacy 2012;3:166-77. [Crossref]
- Qian H, Yang H, Xu W, et al. Bone Marrow Mesenchymal Stem Cells Ameliorate Rat Acute Renal Failure by Differentiation into Renal Tubular Epithelial-Like Cells. Int J Mol Med 2008;22:325-32. [PubMed]
- Jiang WC, Cheng YH, Yen MH, et al. Cryo-Chemical Decellularization of the Whole Liver for Mesenchymal Stem Cells-based Functional Hepatic Tissue Eng. Biomaterials 2014;35:3607-17. [Crossref] [PubMed]
- Wang B, Wang G, To F, et al. Myocardial Scaffold-Based Cardiac Tissue Eng: Application of Coordinated Mechanical and Electrical Stimulations. Langmuir 2013;29:11109-17. [Crossref] [PubMed]
- Krause DS, Theise ND, Collector MI, et al. Multi-organ, Multi-lineage Engraftment by a Single Bone Marrow-derived Stem Cell. Cell 2001;105:369-77. [Crossref] [PubMed]
- Huh D, Kamotani Y, Grotberg JB, et al. Engineering Pulmonary Epithelia and Their Mechanical Microenvironments. In: Khademhosseini A, Borenstein J, Toner M, et al. editors. Micro and Nanoengineering of the Cell Microenvironment. London: Artech House Publishers, 2008:503-33.
- Ziegelaar BW, Aigner J, Staudenmaier R, et al. The Characterisation of Human Respiratory Epithelial Cells Cultured on Resorbable Scaffolds: First Steps Towards a Tissue Engineered Tracheal Replacement. Biomaterials 2002;23:1425-38. [Crossref] [PubMed]
- O'Leary C, Cavanagh B, Unger RE, et al. The Development of a Tissue-engineered Tracheobronchial Epithelial Model Using a Bilayered Collagen-hyaluronate Scaffold. Biomaterials 2016;85:111-27. [Crossref] [PubMed]
- Huang TW, Chan YH, Cheng PW, et al. Increased Mucociliary Differentiation of Human Respiratory Epithelial Cells on Hyaluronan-derivative Membranes. Acta Biomaterialia 2010;6:1191-9. [Crossref] [PubMed]
- Huang TW, Cheng PW, Chan YH, et al. Regulation of Ciliary Differentiation of Human Respiratory Epithelial Cells by the Receptor for Hyaluronan-mediated Motility on Hyaluronan-based Biomaterials. Biomaterials 2010;31:6701-9. [Crossref] [PubMed]
- Forteza R, Lieb T, Aoki T, et al. Hyaluronan Serves a Novel Role in Airway Mucosal Host Defense. FASEB J 2001;15:2179-86. [Crossref] [PubMed]
- Cornelissen CG, Dietrich M, Krüger S, et al. Fibrin Gel as Alternative Scaffold for Respiratory Tissue Eng. Ann Biomed Eng 2012;40:679-87. [Crossref] [PubMed]
- Doolin EJ, Strande LF, Sheng X, et al. Engineering a Composite Neotrachea with Surgical Adhesives. J Pediatr Surg 2002;37:1034-7. [Crossref] [PubMed]
- Albers S, Thiebes AL, Gessenich KL, et al. Differentiation of Respiratory Epithelium in a 3-Dimensional Co-Culture with Fibroblasts Embedded in Fibrin Gel. Multidiscip Respir Med 2016;11:6. [Crossref] [PubMed]
- Risbud M, Endres M, Ringe J, et al. Biocompatible Hydrogel Supports the Growth of Respiratory Epithelial Cells: Possibilities in Tracheal Tissue Engineering. J Biomed Mater Res 2001;56:120-7. [Crossref] [PubMed]
- Brown RA. In the Beginning There were Soft Collagen-cell Gels: Towards Better 3D Connective Tissue Models? Exp Cell Res 2013;319:2460-9. [Crossref] [PubMed]
- Hong HJ, Chang JW, Park JK, et al. Tracheal Reconstruction using Chondrocytes Seeded on a Poly(l-lactic-co-glycolic acid)–fibrin/Hyaluronan. J Biomed Mater Res A Part A 2014;102:4142-50.
- Naito H, Tojo T, Kimura M, et al. Engineering Bioartificial Tracheal Tissue using Hybrid Fibroblast-mesenchymal Stem Cell Cultures in Collagen Hydrogels. Interact Cardiovasc Thorac Surg 2011;12:156-61. [Crossref] [PubMed]
- Kaschke O, Gerhardt HJ, Böhm K, et al. Epithelialization of Porous Biomaterials with Isolated Respiratory Epithelial Cells In Vivo. HNO 1995;43:80-8. [PubMed]
- Yamashita M, Kanemaru SI, Hirano S, et al. Tracheal Regeneration After Partial Resection: A Tissue Eng Approach. Laryngoscope 2007;117:497-502. [Crossref] [PubMed]
- Cortiella J, Nichols JE, Kojima K, et al. Tissue-Engineered Lung: An In Vivo and In Vitro Comparison of Polyglycolic Acid and Pluronic F-127 Hydrogel/Somatic Lung Progenitor Cell Constructs to Support Tissue Growth. Tissue Eng 2006;12:1213-25. [Crossref] [PubMed]
- Vrana NE, Dupret-Bories A, Bach C, et al. Modification of Macroporous Titanium Tracheal Implants with Biodegradable Structures: Tracking In Vivo Integration for Determination of Optimal In Situ Epithelialization Conditions. Biotechnol Bioeng 2012;109:2134-46. [Crossref] [PubMed]
- Vrana NE, Dupret A, Coraux C, et al. Hybrid Titanium/Biodegradable Polymer Implants with an Hierarchical Pore Structure as a Means to Control Selective Cell Movement. PLoS One 2011;6:e20480 [Crossref] [PubMed]
- Idrus RB, Noruddin NA, Cheng CH, et al. Titanium Mesh with Expanded Respiratory Epithelial Cells in Tracheal Reconstruction. J Biomater Tissue Eng 2014;4:367-72. [Crossref]
- Price AP, England KA, Matson AM, et al. Development of a Decellularized Lung Bioreactor System for Bioengineering the Lung: The Matrix Reloaded. Tissue Eng Part A 2010;16:2581-91. [Crossref] [PubMed]
- Stabler CT, Lecht S, Mondrinos MJ, et al. Revascularization of Decellularized Lung Scaffolds: Principles and Progress. Am J Physiol Lung Cell Mol Physiol 2015;309:L1273-85. [PubMed]
- Ott HC, Clippinger B, Conrad C, et al. Regeneration and Orthotopic Transplantation of a Bioartificial Lung. Nat Med 2010;16:927-33. [Crossref] [PubMed]
- Petersen TH, Calle EA, Zhao L, et al. Tissue-Engineered Lungs for In Vivo Implantation. Science 2010;329:538-41. [Crossref] [PubMed]
- Kanzaki M, Yamato M, Hatakeyama H, et al. Tissue Engineered Epithelial Cell Sheets for the Creation of a Bioartificial Trachea. Tissue Eng 2006;12:1275-83. [Crossref] [PubMed]
- Yang J, Yamato M, Kohno C, et al. Cell Sheet Engineering: Recreating Tissues Without Biodegradable Scaffolds. Biomaterials 2005;26:6415-22. [Crossref] [PubMed]
- Grillo HC. Tracheal Replacement: A Critical Review. Ann Thorac Surg 2002;73:1995-2004. [Crossref] [PubMed]
- Zakrzewski JL, van den Brink MR, Hubbell JA. Overcoming Immunological Barriers in Regenerative Medicine. Nat Biotechnol 2014;32:786-94. [Crossref] [PubMed]
- Holtzman MJ, Byers DE, Alexander-Brett J, et al. The Role of Airway Epithelial Cells and Innate Immune Cells in Chronic Respiratory Disease. Nat Rev Immunol 2014;14:686-98. [Crossref] [PubMed]
- Parker D, Prince A. Innate Immunity in the Respiratory Epithelium. Am J Respir Cell Mol Biol 2011;45:189-201. [Crossref] [PubMed]
- Shaykhiev R, Bals R. Interactions Between Epithelial Cells and Leukocytes in Immunity and Tissue Homeostasis. J Leukoc Biol 2007;82:1-15. [Crossref] [PubMed]
- Chen Q, Luo AA, Qiu H, et al. Monocyte Interaction Accelerates HCl-Induced Lung Epithelial Remodeling. BMC Pulm Med 2014;14:135. [Crossref] [PubMed]
- Takizawa H. Airway Epithelial Cells as Regulators of Airway Inflammation Int J Mol Med 1998;1:367-78. (Review). [PubMed]
- Blackwell TS, Hipps AN, Yamamoto Y, et al. NF-κB Signaling in Fetal Lung Macrophages Disrupts Airway Morphogenesis. J Immunol 2011;187:2740-7. [Crossref] [PubMed]
- Savill JS, Wyllie AH, Henson JE, et al. Macrophage Phagocytosis of Aging Neutrophils in Inflammation. Programmed Cell Death in the Neutrophil Leads to its Recognition by Macrophages. J Clin Invest 1989;83:865-75. [Crossref] [PubMed]
- Erjefält JS, Erjefält I, Sundler F, et al. In Vivo Restitution of Airway Epithelium. Cell Tissue Res 1995;281:305-16. [Crossref] [PubMed]
- Chamoto K, Gibney BC, Lee GS, et al. Migration of CD11b+ Accessory Cells During Murine Lung Regeneration. Stem Cell Res 2013;10:267-77. [Crossref] [PubMed]
- Lambrecht BN, Hammad H. The Role of Dendritic and Epithelial Cells as Master Regulators of Allergic Airway Inflammation. Lancet 2010;376:835-43. [Crossref] [PubMed]
- Polito AJ, Proud D. Epithelial Cells as Regulators of Airway Inflammation. J Allergy Clin Immunol 1998;102:714-8. [Crossref] [PubMed]
- Skerrett SJ, Liggitt HD, Hajjar AM, et al. Respiratory Epithelial Cells Regulate Lung Inflammation in Response to Inhaled Endotoxin. Am J Physiol Lung Cell Mol Physiol 2004;287:L143-52. [Crossref] [PubMed]
- Kato A, Schleimer RP. Beyond Inflammation: Airway Epithelial Cells are at the Interface of Innate and Adaptive Immunity. Curr Opin Immunol 2007;19:711-20. [Crossref] [PubMed]
- Schleimer RP, Kato A, Kern R, et al. Epithelium: At the Interface of Innate and Adaptive Immune Responses. J Allergy Clin Immunol 2007;120:1279-84. [Crossref] [PubMed]
- Ryu JH, Kim CH, Yoon JH. Innate Immune Responses of the Airway Epithelium. Mol Cells 2010;30:173-83. [Crossref] [PubMed]
- Xing Z, Ohtoshi T, Ralph P, et al. Human Upper Airway Structural Cell-derived Cytokines Support Human Peripheral Blood Monocyte Survival: A Potential Mechanism for Monocyte/Macrophage Accumulation in the Tissue. Am J Respir Cell Mol Biol 1992;6:212-8. [Crossref] [PubMed]
- Churchill L, Friedman B, Schleimer RP, et al. Production of Granulocyte-Macrophage Colony-Stimulating Factor by Cultured Human Tracheal Epithelial Cells. Immunology 1992;75:189-95. [PubMed]
- Smith SM, Lee DKP, Lacy J, et al. Rat Tracheal Epithelial Cells Produce Granulocyte/Macrophage Colony-stimulating Factor. Am J Respir Cell Mol Biol 1990;2:59-68. [Crossref] [PubMed]
- Ohtoshi T, Vancheri C, Cox G, et al. Monocyte-Macrophage Differentiation Induced by Human Upper Airway Epithelial Cells. Am J Respir Cell Mol Biol 1991;4:255-63. [Crossref] [PubMed]
- Ghaemmaghami AM, Hancock MJ, Harrington H, et al. Biomimetic Tissues on a Chip for Drug Discovery. Drug Discov Today 2012;17:173-81. [Crossref] [PubMed]
- Rahim R, Manuel O, Amy D, et al. A Janus-paper PDMS Platform for Air–Liquid Interface Cell Culture Applications. J Micromech Microeng 2015;25:055015 [Crossref]
- Bérubé K, Pitt A, Hayden P, et al. Filter-well Technology for Advanced Three-Dimensional Cell Culture: Perspectives for Respiratory Research. Altern Lab Anim 2010;38:49-65. [PubMed]
- Huang S, Wiszniewski L, Constant S. The Use of In Vitro 3D Cell Models in Drug Development for Respiratory Diseases. In: Kapetanović IM. editor. Drug Discovery and Development - Present and Future. InTech, 2011.
- Zhu Y, Chidekel A, Shaffer TH. Cultured Human Airway Epithelial Cells (Calu-3): A Model of Human Respiratory Function, Structure, and Inflammatory Responses. Crit Care Res Practe 2010;2010:8.
- Florea BI, Cassara ML, Junginger HE, et al. Drug Transport and Metabolism Characteristics of the Human Airway Epithelial Cell Line Calu-3. J Control Release 2003;87:131-8. [Crossref] [PubMed]
- Forbes B, Ehrhardt C. Human Respiratory Epithelial Cell Culture for Drug Delivery Applications. Eur J Pharm Biopharm 2005;60:193-205. [Crossref] [PubMed]
- Bérubé K, Prytherch Z, Job C, et al. Human Primary Bronchial Lung Cell Constructs: The New Respiratory Models. Toxicology 2010;278:311-8. [Crossref] [PubMed]
- Klein SG, Hennen J, Serchi T, et al. Potential of Coculture In Vitro Models to Study Inflammatory and Sensitizing Effects of Particles on the Lung. Toxicol In Vitro 2011;25:1516-34. [Crossref] [PubMed]
- Müller L, Riediker M, Wick P, et al. Oxidative Stress and Inflammation Response after Nanoparticle Exposure: Differences Between Human Lung Cell Monocultures and an Advanced Three-dimensional Model of the Human Epithelial Airways. J R Soc Interface 2010;7:S27-40. [Crossref] [PubMed]
- Harrington H, Cato P, Salazar F, et al. Immunocompetent 3D Model of Human Upper Airway for Disease Modeling and In Vitro Drug Evaluation. Mol Pharm 2014;11:2082-91. [Crossref] [PubMed]
- Castillon N, Avril-Delplanque A, Coraux C, et al. Regeneration of a Well-differentiated Human Airway Surface Epithelium by Spheroid and Lentivirus Vector-transduced Airway Cells. J Gene Med 2004;6:846-56. [Crossref] [PubMed]
- Lee J-H, Bhang Dong H, Beede A, et al. Lung Stem Cell Differentiation in Mice Directed by Endothelial Cells via a BMP4-NFATc1-Thrombospondin-1 Axis. Cell 2014;156:440-55. [Crossref] [PubMed]
- Esch EW, Bahinski A, Huh D. Organs-on-Chips at the Frontiers of Drug Discovery. Nat Rev Drug Discov 2015;14:248-60. [Crossref] [PubMed]
- Bhatia SN, Ingber DE. Microfluidic Organs-on-Chips. Nat Biotechnol 2014;32:760-72. [Crossref] [PubMed]
- Sallin P, Barbe L, Gazdhar A, et al. Lung on Chip: In vitro HGF Effects on Injured Alveolar A549 Epithelial Cells in Microfluidic System. Eur Respir J 2014;38:4584.
- Blume C, Reale R, Held M, et al. Temporal Monitoring of Differentiated Human Airway Epithelial Cells Using Microfluidics. PLoS One 2015;10:e0139872 [Crossref] [PubMed]
- Huh D, Leslie DC, Matthews BD, et al. A Human Disease Model of Drug Toxicity–Induced Pulmonary Edema in a Lung-on-a-Chip Microdevice. Sci Transl Med 2012;4:159ra147 [Crossref] [PubMed]
Cite this article as: Kumar P, Vrana NE, Ghaemmaghami AM. Prospects and challenges in engineering functional respiratory epithelium for in vitro and in vivo applications. Ann Cardiothorac Surg 2017;1:2.


