
Microphysiological Systems: automated fabrication via extrusion bioprinting
Introduction
In 1965, Gordon Moore predicted computing would increase in power and decrease in cost at an exponential rate. While this law has continued to uphold in computing, advances in drug development have followed a troubling alternative trend, referred to as EROOM’s law (1). Since 1950, the number of novel treatments approved per billion US dollars spent on R & D has halved approximately every 9 years (1). A large factor in EROOM’s law is the high failure rate of potential therapies in clinical trials, highlighting the ineffectiveness of current in vitro testing methods. Many current in vitro models use static 2D cultures that bear little resemblance to native tissue environments. In 2015, less than 12% of drugs entering clinical trials resulted in an approved medicine, costing millions of dollars and exposing patients to harmful side effects (2). MPS models have provided great promise for improving the rate of innovation in drug development and off-setting EROOM’s law through more physiologically relevant in vitro models (3). These systems are designed to recapitulate the functions of living tissues or organs through relevant microarchitecture and dynamic stimulation of cultures (4).
The fabrication of these devices involves two main parts: microfabrication of the tissue cultures and design of the microfluidics, sensors and other dynamic systems for the delivery of nutrients, chemicals, biological factors and mechanical or electrophysiological signals in a controlled microenvironment. Several techniques are available for this fabrication process, including photolithography, soft lithography, dry etching, sputter coating, chemical vapor deposition and stereolithography (4). While each of these fabrication methods provides individual advantages, many involve non-biocompatible hazardous reagents and require laborious multi-step processes (4).
Recently, extrusion biofabrication methods have been applied for the fabrication of MPS. Bioprinting technologies allow scientists to pattern cell types and create more complex tissue structures, creating more physiologically relevant models useful in translational research (5). This biocompatible process allows the potential for a single-step, automated fabrication of MPS, creating a more efficient fabrication process. Additionally, bioprinting allows for the integration of 3D tissue designs within MPS devices, as opposed to 2D cultures often used with other methods. This review article details design components and considerations for these devices, recent advances in MPS fabricated through extrusion biofabrication and future considerations for this technology.
3D biofabrication technologies
Advantages of 3D culture, such as cell adhesive sites in three dimensions and tunable mechanical properties, became apparent with the development of spheroid cultures in the 1970s (6). As research on 3D cultures progressed, technologies were developed for increased control over the 3D microenvironment. The development of the first 3D bioprinter in the 1990s allowed scientists to design more intricate 3D microarchitectures (7,8). Since the 1990s, biofabrication technologies have become increasingly complex. Today there are a variety of biofabrication methods available, which can be split into two general groups: jetting-based and extrusion-based bioprinting (9).
Jetting-based bioprinting involves non-contact techniques to fabricate 2D or 3D structures using picolitre bioink droplets layered onto a substrate (9). Common jetting-based methods include piezoelectric, thermal, pneumatic and laser-assisted technology (9). Piezoelectric actuators apply a piezo-crystal pulse to eject a small droplet of a bioink (7,9). Thermal methods create bioink droplets by locally increasing temperature within the bioink compartment to generate a bubble (9). Pneumatic pressure and valves generate droplets through opening and closing of microvalves (9). Focused laser energy methods generate vaporization through laser systems to produce small droplets (9). Jetting-based technologies offer advantages such as high resolution ranging from 20–100 µm, but are limited to low viscosity bioinks and have a long processing time, limiting the size of structures that can be created with these methods (7,9).
Extrusion-based bioprinting dispenses continuous filaments of bioinks through micro-nozzles to build 2D or 3D structures (9). These methods dispense bioinks through pneumatic pressure or syringe pumps (9). The speed and volume of material dispensed can be adjusted by controlling the pressure level or displacement of the piston or pump (7,9). While these methods offer lower resolution compared to jet-based technologies, they allow for the fabrication of constructs with clinically relevant sizes in a realistic time-period and provide versatility in material compatibility. Because of these advantages, extrusion-based bioprinting is often regarded as the most promising and is the most common biofabrication method used to develop 3D microphysiological systems (MPS) (4,7,10,11). A more detailed review on various biofabrication technologies can be found in Seol et al. (9).
MPS design components and considerations
The components of MPS can be broken into four key elements: a microfluidic chip or culture chamber, live microtissues, components for dynamic culture and sensors for results readout (12). A visualization of these systems is demonstrated in Figure 1.
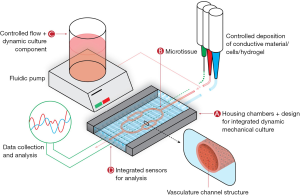
Microfluidic housing/culture chamber
The microfluidic chip contains the housing, or culture chamber, for the live microtissues and often connects this chamber to a dynamic flow system for perfusion of nutrients, bioactive molecules and drug compounds to the live microtissues. Ideally, a culture chamber provides a sterile environment that can be fabricated or molded into custom geometries and can be easily analyzed noninvasively. For this portion of the device, many microfluidics have utilized silicone-based inks such as poly(dimethylsiloxane) (PDMS), which are highly compatible with a variety of fabrication methods and provide optical clarity, low cost and high reproducibility (13). However, PDMS comes with some significant disadvantages for MPS including deformation, evaporation, absorption, leaching and hydrophobic recovery (13-15). The most prominent disadvantage of this material for use in MPS is the absorption of proteins, drugs and hydrophobic molecules, complicating the pharmacokinetic and pharmacodynamic models of drugs. A variety of alternatives to PDMS have been developed, with some of the most promising including acrylonitrile butadiene styrene (ABS), poly(lactic acid) (PLA) and polycaprolactone (PCL). These materials, which overcome the disadvantages of deformation, absorption, leaching and hydrophobic recovery with PDMS, can be used with transparent glass or polystyrene substrates to fabricate chambers for MPS. Additionally, because of their printability, these materials can be used in extrusion biofabrication platforms for one step biofabrication of both the culture chamber and the live microtissues. A summary of these materials, along with advantages and disadvantages, is presented in Table 1. Many recent publications have attempted to move away from the use of PDMS towards these alternative materials.
Table 1
| Material | Advantages | Disadvantages | Ref. |
|---|---|---|---|
| PDMS/silicone-based inks | Compatible with variety of fabrication methods; optically transparent; non-degradable | Deformation; absorption of hydrophobic drugs; leaching; hydrophobic recovery | (10,15-19) |
| PCL | Biocompatible; less prone to bulk absorption of hydrophobic drugs; low melt temperature; rigid | Not optically transparent | (20,21) |
| ABS | Biocompatible; less prone to bulk absorption of hydrophobic drugs; rigid | Not optically transparent; high melt temperature | (15) |
| PLA | Biocompatible; less prone to bulk absorption of hydrophobic drugs; rigid | Not optically transparent; high melt temperature | (15) |
The microfluidic chip contains the housing, or culture chamber, for the live microtissues and often connects this chamber to a dynamic flow system for perfusion of nutrients, bioactive molecules and drug compounds to the live microtissues. While PDMS is the most common material used for this component, disadvantages including absorption of hydrophobic drugs have led to the development of alternative housing materials, including photocurable resins, PCL, ABS and PLA (
Live microtissues
The primary component of any MPS is the live microtissue. Live microtissues can vary in complexity, from simple 2D cultures along the walls or grooves of the microfluidic chip to intricate, multicellular 3D microarchitectures. Extrusion biofabrication offers the capability to create both simple, uniform cell layers and more complex 3D architectures. A variety of tissue types have been modeled as MPS through extrusion bioprinting, some of which are detailed in Section IV below and in Table 2.
Table 2
| Tissue/disease model | Cell type(s) | Materials | Housing material | Length of culture | Analysis | Culture method | Ref. |
|---|---|---|---|---|---|---|---|
| Liver | HepG2/C3A Spheroids | GelMA | PDMS/PMMA and glass slide bottom | 30 days | Hepatocytes responsive oxygen concentration and shear stress; |
Bioreactor platform (custom design) with peristaltic syringe pump | (10) |
| Liver | HepG2, HUVECs | Gelatin and 2% Collagen type I | PCL with glass slide bottom | 6 days | Quantitative analysis of albumin and viability | Perfusion with peristaltic pump | (20) |
| Liver | Cryopreserved hepatocytes spheroid; Zucker fatty rat hepatocytes spheroids | Scaffold-free | PDMS/PMMA and glass slide bottom | 77 days, 23 days (rat) | Measured gene expression, glucose production response to insulin, and bile acid secretion | Cultured in perfusion chamber for 4 days with syringe pump, then cultured with moderate rocking or shaking for more than 2 weeks | (19) |
| Heart | HUVECs (bioprinted); neonatal rat cardiomyocytes hiPSC-CMs | Alginate, GelMA | PDMS/PMMA gaskets | 33 days | Live/dead staining; |
Perfusion with peristaltic pump | (17) |
| Heart | hiPS-CMs, NRVMs | Dextran ink, TPU ink, CB: TPU ink, Ag: Pa ink | Soft PDMS ink, Rigid PDMS ink, PLA, ABS | 28 days (hiPS-CMs) | Quantified resistance changes through custom connected device; |
Static culture with built in sensors for electronic readout of contractile stresses | (15) |
| Kidney | Human immortalized PTECs, A498 renal cancer cells, HNDF | Fugitive pluronic, gelatin-fibrin matrix | Silicone elastomer with glass base | 65 days | Albumin uptake; |
Perfusion of continuous, unidirectional flow with peristaltic pump | (11) |
| Nervous System | Schwann cells, hippocampal neurons cell suspensions | Scaffold-free | Silicone, Polycaprolactone in combination with glass dishes | 14 days | Response to infection of PRV | Static culture | (21) |
| Vessels (thrombosis model) | HUVECs, HNDFs | GelMA, pluronic F127 | Petri dish | 7 days | Formation of thrombi; response to thrombolytic agent | Perfusion with syringe pump | (22) |
| Cancer | Human breast adenocarcinoma cell line (MDA-MB-231) | Scaffold-free | PDMS | 14 days | Cell proliferation and morphology | Perfusion with syringe pump | (18) |
| Lung | Human alveolar epithelial type II cell line A549, EA.hy926 hybrid human cell line (human umbilical vein endothelial cells fused with A549 cells) | BD matrigel BM matrix growth factor-reduced | Millicell-CM organotypic tissue culture plate inserts | 3 days | Comparison of manually seeded and bioprinted monocultures; |
Static culture on Millicell-CM organotypic tissue culture plate inserts | (23) |
| Brain | Mouse brain endothelial cell line (bEnd.3) | Collagen hydrogel | PMMA plate with glass bottom | 21 days | Characterization of cell adhesion and construct permeability, diffusivity; disruption of barrier with mannitol | Laminar flow with syringe pump (0.1 mm/s) | (16) |
A variety of tissue models have been developed via extrusion bioprinting. This table details some of the MPS fabricated with this method, including the cell types, microtissue materials, housing materials, length of culture, analysis and culture methods utilized in each model. HNDF, human neonatal dermal fibroblasts; PMMA, poy(methyl methacrylate); PDMS, poly(dimethylsiloxane); PCL, polycaprolactone; ABS, acrylonitrile butadiene styrene; PLA, poly(lactic acid); MPS, Microphysiological systems; GelMA, gelatin methacrylate; HUVECs, human umbilical vein endothelial cells; NRVMs, neonatal rat ventricular myocytes; hiPS-CMs, human induced pluripotent stem cell derived cardiomyocytes; TPU, thermoplastic polyurethane; CB, carbon black nanoparticles; BD, Becton Dickinson; BM, basement membrane.
3D Microtissues
Biofabrication of MPS allows for spatial control of cell-laden materials to mimic multi-cellular microphysiological architectures. A variety of materials can be used for this purpose. Briefly, common materials used in 3D microtissues for cell deposition include naturally-derived and synthetic hydrogels. While some MPS utilize scaffold-free cell slurries, matrix bioinks allow for enhanced resolution and control over cell deposition. Additionally, use of matrix bioinks allows for control over substrate mechanical properties and biological factors. This material and cell selection are not the primary focus of this review, although more can be learned from our previous publication on this topic (8). Some examples of matrix bioinks used in MPS can be found in Table 2.
Channel fabrication in live 3D microtissues
Vasculature is a major component of many tissues and is critical in modeling physiologically relevant in vitro models. Vasculature is particularly important for drug screening technologies, as many compounds are injected directly into the blood stream. Many MPS incorporate some type of channel to mimic vascular flow, ranging from simple straight channels to more complex branched features.
Extrusion biofabrication offers a variety of methods for incorporation of microchannels to apply flow and recapitulate vasculature structures within microtissues (4,11,22,24). Often, sacrificial bioinks are used to fabricate complex channels within a microtissue. Some common materials used for this purpose are pluronic F127, agarose, gelatin and carbohydrate glass (8). Bioinks such as pluronic, agarose and gelatin offer a thermal reversible gelation that allow the materials to be printed at room temperature then washed away either through heating or cooling. Bioinks such as carbohydrate glass offer increased mechanical stiffness compared to other softer sacrificial materials that allow for easier construction of more complex designs (8).
Temporary support baths utilizing the sacrificial material gelatin have also demonstrated great promise for the fabrication of microtissues with complex channels (8). The FRESH method, further described in a previous publication, provides temporary support of soft, fragile bioinks during fabrication. This method has not yet been used for the fabrication of MPS, but has great potential for the automation of highly complex vasculature (8,25).
Dynamic culture: flow, mechanical and electrical stimulation
MPS may also contain extra components to stimulate flow or incorporate additional mechanical or electrical stimulation of tissues during culture.
Stimulating flow: pumps for MPS
To create better in vitro models of dynamic in vivo cellular systems, pumps are often used to expose cells to flowing media, as compared to static in vitro cultures. Pumps can also act as an automated way to change cell media, and can be either “closed” (recirculating liquid) or “open” (refillable main liquid reservoir) systems. The application should determine the type of pump used, as different types offer various advantages and disadvantages. Some characteristics to consider are flow rate, flow type (pulsatile or constant), duration of flow, mechanical effects, and electrical requirements. The most common pumps used in MPS are gravity-driven flow, peristaltic pumps, syringe pumps and electroosmotic pumps (Table 3, Figure 2). For a more comprehensive review on pumps, refer to reference (26).
Table 3
| Pump | Advantages | Disadvantages | References |
|---|---|---|---|
| Gravity-driven flow | Simple, inexpensive; no power required; prevents air bubble formation; works in open or closed systems | Cannot produce pulsatile flow; difficult to control | (26,27) |
| Peristaltic pumps | Produces pulsatile and reversible flow; works in open or closed systems | Power required; mechanical parts can have large footprint | (24,26,28) |
| Syringe pumps | Produces pulsatile or constant flow; fine control of flow | Power required; mechanical parts can have large footprint; only works in closed systems | (22,29,30) |
| Electroosmotic pumps | No mechanical parts; reversible flow | Power required; high voltages can cause cell lysis; only works in open systems | (27,31) |
To create better
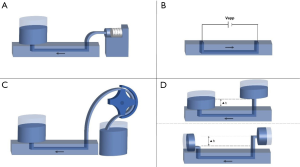
Gravity-driven flow
Gravity-driven flow is a passive flow technique that is simple to implement, as it requires no mechanical parts or power. It is comprised of only two liquid reservoirs with a height difference between them, creating a flow of liquid from the higher reservoir to the lower one (Figure 2). In a closed system, the flow rate will decrease as the height difference between the reservoirs decreases. However, this effect can be minimized by orienting the reservoirs horizontally (Figure 2). In an open system, refilling the higher reservoir will also negate this problem and prolong the flow. While gravity-driven pumps are only able to produce a constant flow rate, the simplicity of these pumps can be beneficial in complex applications where tubing and multiple pumps would otherwise be needed.
Peristaltic pumps
Peristaltic pumps are a type of active, positive-displacement pump that results in sinusoidal, pulsatile flow (24,26). With these systems, liquid is pushed through a tube that is wrapped around rotors that are turned by a rotating shaft (Figure 2). Peristaltic pumps are very versatile as they are bi-directional, have large ranges of flow rates, and can be used as either closed or open systems. However, peristaltic pumps require power and have mechanical components that can wear with time. Additionally, these systems can have a large footprint, which brought about the push for smaller pumps such as the peristaltic micropump (28). Wagner et al. utilized a peristaltic micropump in a multi-organ-chip system that was integrated directly onto the MPS (32). This pump, no more than a few millimeters in width, provided pulsatile flow at a circulation rate of 40 µL/min to two culture compartments (32). Continued development and miniaturization of peristaltic pumps provides great promise for the advancement of MPS.
Syringe pumps
Another type of active, positive-displacement pump is the syringe pump (22,29,30). These pumps utilize a motor-driven piston to create either pulsatile or constant flow stemming from a syringe full of liquid (Figure 2). Since all liquid must originate from the syringe, it is a closed system. This can become an obstacle in longer term projects where the piston syringe would need to be refilled. However, these pumps offer significant control over flow, making them very popular and widely available.
Electroosmotic pumps
As opposed to many other pumps, electroosmotic pumps are not mechanical, meaning that they have no moving parts. Instead, these pumps use electricity for active flow generation in microchannels in an open system. A surface-ionizable material, such as silica, becomes negatively charged due to deprotonation (31). This charge attracts cations, which form a “positively charged solution layer” close to the channel’s surface. When an external electric field is applied, the cations move, pulling the solution with them. The opposite can be done with a positively charged surface. Electroosmotic flow (EOF) is simple but requires caution, as electric fields above 1 kV/cm can cause cell lysis (27).
Electrical stimulation and integrated sensors
A variety of biocompatible conductive materials have been used to provide electrical stimulation to microtissues (33,34). Electroactive biomaterials have the ability to deliver electrical, electrochemical and electromechanical stimulation to cells and can also be used to create integrated sensors in MPS (35). Bioprinting provides the opportunity to incorporate these materials directly into microtissues and MPS. Some common conductive materials utilized in bioprinting include gold, graphene, carbon and silver-based inks as well as electrically conductive polymers such as polymer polypyrrole (PPy).
Gold nanoparticles are commonly incorporated into biomaterials for a variety of applications and offer an array of benefits, including high surface energy, high conductivity, and biocompatibility (36). Gold-based materials have been utilized for the fabrication of biosensors and have been incorporated into live microtissues for functionalization and electrical stimulation. A gold nanorod-incorporated (GNR) gelatin methacrylate (GelMA)-based ink was used to print functional cardiac tissue constructs. These GNR constructs demonstrated increased cell adhesion and organization as well as promotion of synchronized contraction of cardiac cells compared to non-GNR constructs (37).
Due to its unique structure, graphene provides a variety of advantages including high fracture strength, electrical and thermal conductivity, fast mobility of charge carriers and high biocompatibility (38). This material was incorporated into a GelMA-based ink to fabricate graphene nanoplatelets for nerve tissue regeneration (39). The conductive substrate provides cues to developing cells to reinforce electrical connections and promotes formation of a neural network (39).
Carbon-based inks are another popular material for biofabrication due to their electrical conductivity and mechanical properties. Several carbon-based bioinks have been fabricated over the past year, but a particularly promising one from Adams et al. demonstrates a biocompatible carbon-nanotube ink that can fabricate 3D functionalized structures (40). This bioprinted MPS demonstrated high cell attachment and differentiated actin cytoskeletal structures when electrical impulse was applied compared to unstimulated scaffolds (40).
Recently, electroconductive materials have also been used in MPS to incorporate sensors for automated, quantifiable results readout. Extrusion biofabrication presents the opportunity to fabricate these sensors directly into the live microtissues, as described in the cardiac MPS from Lewis et al. shown in Section IV and Figure 3 below (15). This system utilized a silver particle-filled, polyamide (Ag:PA) ink to fabricate electrical leads and contact pads, as well as a TPU-CB-TPU (TPU, thermoplastic polyurethane; CB, carbon black nanoparticles) ink within the structures (15). These sensors allowed for noninvasive analysis of the MPS (15).
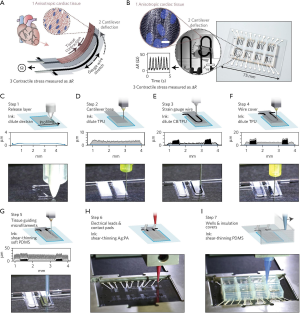
Electroactive biomaterials have also been used for mechanical stimulation in MPS. Svennersten et al. utilized a PPy, which exhibits good biocompatibility, chemical stability and high conductivity, to fabricate a microactuator system on a chip that delivered mechanical stimulation to renal epithelial cells (35,41). This stimulation resulted in increased internal calcium levels, a documented response to mechanical stimulation (35).
Mechanical stimulation
Many tissues are exposed to continuous mechanical stimulation in vivo. To properly mimic this environment, many MPS aim to include mechanical stimulation (42,43). In addition to use of electroactive biomaterials, this stimulation can also be achieved through application of uniaxial, cyclical, or biaxial strain.
Huh et al. successfully applied uniaxial strain in a lung on a chip model through generation of displacement along one axis (43,44). This model incorporated 3D microarchitecture and dynamic mechanical activity to recapitulate the function of the alveolar-capillary interface (44). In a different study, cyclical stain was used to promote differentiation of murine embryonic stem cells into cardiomyocytes, demonstrating upregulation of α-MHC in mechanically stimulated embryoid bodies (43). While sometimes more complex than uniaxial or cyclical strain, biaxial strain has high potential for high-throughput devices and can be incorporated in a variety of ways. Biaxial strain was applied with a stretching device and applied vacuum chamber on a lung-on-a-chip device (43). Alternatively, another model designed wells with a deformable bottom to apply biaxial strain to cardiac progenitor cells, demonstrating a successful activation of β-catenin (43).
Mechanically active MPS have the potential to reproduce dynamic physical forces that are critical for organ function and disease development. To effectively recapitulate in vivo environments, careful consideration should be made in designing these forces. A more comprehensive review of these forces and methods to apply them can be found in reference (45). Biofabrication platforms have the potential to simplify the fabrication of these incorporated components through the automation of chamber designs used to apply these forces.
MPS fabricated via extrusion bioprinting
Utilizing the components described above, many MPS of varying tissues have been fabricated through extrusion biofabrication. Some of these models are summarized in Table 2.
Brain-on-a-chip
The blood-brain barrier (BBB) plays an integral role in regulating transport phenomena of small- and macro-molecules as well as maintaining homeostasis of the brain microenvironment (16). Dysfunction of the BBB is associated with a variety of neurological disorders, including neurodegenerative disease and ischemia, and as such, there is a high interest in developing more effective in vitro models of this system (16). Kim et al. developed a model by 3D co-printing a polymeric frame with dissolvable poly(vinyl alcohol) to create a microvasculature structure to encase cell-laden collagen. This model was used to develop a time-dependent evolution of the barrier function for up to 3 weeks (16).
Heart-on-a-chip
Cardiovascular disease prevails as the leading cause of death worldwide (46). Additionally, a large number of treatments for other diseases present cardiotoxic side effects, leading to withdrawal of therapies from the market (46). While a biomimetic cardiac model is clearly necessary for the advancement of medicine, the complex geometry and mechanisms of the heart is especially difficult to recapitulate and requires advanced fabrication methods.
Vasculature in cardiac models is necessary for the culture of thick cardiac tissue, but its complexity is not able to be replicated through traditional fabrication methods. Zhang et al. explored a novel strategy for fabricating endothelialized myocardial tissues via the use of extrusion bioprinting, microfluidics and stem cells (17). When combined with a microfluidic perfusion bioreactor, the bioprinted model remained viable for up to 15 days with perfusion, compared to significant cell death by day 7 without perfusion (17). This model was also tested with hiPSC-cardiomyocytes and with further development has a potential for use in personalized drug screening (17).
While 3D models provide enhanced physiological relevance, the increased complexity often causes challenges when analyzing tissue constructs. The Lewis and Parker group worked to integrate embedded sensors into a 3D bioprinted cardiac MPS for easier analysis of contractile forces of cells (15). Using a custom extrusion biofabrication platform, the group was able to sequentially print multiple functional materials for a one-step fabrication of a comprehensive chip to test contractile force and drug response of cardiac tissues (15). The constructs were fabricated with an array of PDMS wells as the microfluidic housing with a sandwich structure of TPU-CB-TPU printed inside each well. The electrical conductivity of the embedded CB structures is affected by strain and was used to measure the contractile forces of cells cultured on the constructs (15).
Kidney-on-a-chip
While 20% of failures in Phase III clinical trials are caused from renal toxicity, only 2% of drugs tested in pre-clinical phases fail from this issue (11). Therefore, it is imperative to create more physiologically relevant 3D models of the kidney tissue to improve pre-clinical testing accuracy of therapeutics and drugs (11). The proximal tubule, responsible for 65–80% of nutrient absorption and transport from the renal filtrate to the blood, is the most frequently damaged site in the kidney from prescription drugs (11). Prior to the study by Homan et al., models of the proximal tubule consisted of either 3D static cultures limited to 1 mm in size or perfusion models of 2D culture (11). Through extrusion bioprinting, this group was able to fabricate complex 3D perfusable architecture that was cultured for over 2 months. The model was tested with a nephrotoxin, cyclosporine A, and demonstrated disruption of the epithelial barrier in a dose dependent manner (11). This model significantly enhanced epithelial morphology and functional properties relative to the same cell types grown on 2D controls or without perfusion. The automated, customizable, one-step fabrication method demonstrates the power of extrusion biofabrication for modeling MPS (11).
Liver-on-a-chip
Drug-induced-liver-injury (DILI) is the most common reason for regulatory actions post drug approval, yet almost 50% of drugs that cause substantial DILI produce little or no toxicity in animal or preclinical models (47,48). In vitro liver models have increased in complexity over the past few decades, from simple organoids of hepatocytes to 3D patterned cells in microfluidic chambers (49). Comparisons of various models have demonstrated the superiority of 3D cultures as well as fluid flow over 2D, static cultures (48-50). Extrusion biofabrication presents the capability to create advanced 3D architectures and culture chambers in one step, and a variety of liver-on-chip models have been fabricated through this method (51-53).
Bhise et al. utilized extrusion biofabrication to control deposition, placement and thickness of spheroid-laden GelMA solutions to fabricate a 3D hepatic construct (10). Through bioprinting technology, variables such as spheroid concentration, hydrogel composition and geometry of printed constructs could be easily changed and controlled (10). The biofabrication process was designed to interact with a pre-fabricated bioreactor and culture chamber, which was used to provide a controlled flow rate of media to constructs at 200 µL/h (10) Cultured spheroids maintained viability for up to 30 days of culture and demonstrated similar response to APAP treatment as animal models, demonstrating potential for its use as a model for drug toxicity analysis (10).
Lee et al. not only created geometrically complex multicellular constructs, but also utilized extrusion bioprinting for a one-step fabrication process of the construct and culture chamber (20). The group tested various geometries, including a 2D printed scaffold-free model, a single-cell 3D model and a multi-cellular 3D model, all encased in a 3D printed polycaprolactone or PDMS chamber (20). Results demonstrated lower protein absorption in PCL chamber constructs compared to platforms fabricated with PDMS, and liver function was significantly improved in 3D models compared to 2D ones (20) .
Lung-on-a-chip
One of the most common methods of entry for drugs, pathogens and other agents into the human body are the lungs. Modeling this process via MPS is important for studying disease pathways and to analyze the transport of aerosol based drugs (49). Most systems aim to model the epithelial-endothelial layers that create a semi-permeable barrier that separate air and fluid channels (49).
The automation of fabricating simple cell layers through biofabrication was demonstrated by Horváth et al. by printing cell-laden Matrigel layers of human alveolar epithelial type II cell line A549 and hybrid human cell line EA hy926 onto transwell culture plate inserts (23). Compared to constructs fabricated via pipetting, the cultures fabricated via extrusion bioprinting demonstrated improved uniformity and tightness of mono- and co-cultures (23). This automated fabrication process, combined with pneumatic channels and cyclic wall stretching used in Huh et al., described in Section III above, could create a truly superior model for analysis (44). Additionally, the chambers utilized in Huh et al. could be potentially bioprinted as well, creating a simplified, one-step fabrication process (44).
Nervous-system-on-a-chip
Enhanced models of the nervous system are necessary to better understand the complex neurological phenomena and to develop effective treatment for neurological disorders. Johnson et al. developed a bioprinted nervous system model through fabricated PCL microchannels with compartmentalized grease and silicone chambers and cell suspensions of rat embryonic hippocampal neurons, rat embryonic sensory neurons, rat embryonic Schwann cells and porcine kidney epithelial cells (21). These components migrated throughout the channels to form an interconnected nervous system that was used to investigate the preferential transmission of pseudorabies virus (PRV) from the cell body of PNS, CNS or the terminal site (21).
Vessels-on-chips
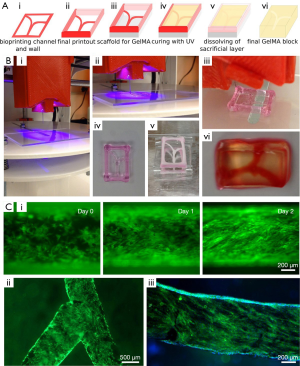
In vitro models focused on vascular-related diseases are integral for developing improved treatments of these diseases. Thrombosis in particular is a leading cause for morbidity and mortality from cardiovascular diseases (22). Through the commercially available Allevi (previously BioBots) platform, Zhang et al. fabricated a fibroblast laden GelMA hydrogel with endothelialized channels and induced thrombi through the infusion of blood into the model (22). Migration of cells in response to the clot and the pathology of fibrosis were analyzed with this model. To form the vascular channels, Zhang et al. utilized pluronic F127, a sacrificial material that can be washed away after fabrication (22). This model is illustrated in Figure 4 (22).
Cancer-on-chips
In addition to healthy tissue models, there is also a large need for disease models for in vitro testing. Tumor MPS have the potential to offer more physiologically relevant models through tumor heterogeneity, vasculature incorporation and biomimetic spheroid structures (18). Bioprinting has demonstrated improvement over previous fabrication methods for cell deposition into microfluidic devices, and offers great potential for advanced cancer in vitro models (18).
Multi-tissue platforms: towards “Body-on-a-chip” devices
The advancement of single MPS platforms has led to research on multi-tissue devices towards full “body-on-a-chip” or “human-on-a-chip” devices. These devices aim to create more systemic in-vitro models of metabolic pathways within the human body for more relevant pharmacokinetic and pharmacodynamic analysis.
One notable multi-tissue MPS fabricated through extrusion bioprinting is a three-tissue organ-on-a-chip model from Skardal et al. (54). To mimic inter-organ responses and create a multi-tissue biomimetic model, Skardal et al. developed a three-tissue MPS consisting of liver, heart and lung microtissues connected in a closed, circulatory perfusion system. The system demonstrated secondary toxicity in cardiac tissues likely caused from the release of inflammatory cytokines from lung microtissue after exposure to a known toxic compound bleomycin (54). Skardal et al. utilized biofabrication to construct liver structures and the PCL microchambers (54).
While few multi-tissue systems have been developed through biofabrication, there is high potential for advancement of multi-tissue systems with this method, which lends easily to the scaled complexity of these systems (55,56). A more comprehensive review of other multi-tissue MPS can be found in reference (57).
Discussion: current limitations and future considerations
Advances in biofabrication technologies, specifically in extrusion biofabrication, have led to the progression of increasingly biomimetic MPS. Extrusion biofabrication offers many advantages over other methods, including biocompatibility, the versatility to pattern a variety of materials, and the automated one-step fabrication of multi-material, complex microstructures. These advantages have led to the development of superior tissue models previously impossible to fabricate through other methods. These models incorporate 3D cell culture, advanced sensors for analysis and dynamic culture of systems through controlled flow and electrical and mechanical forces.
Yet many fabricated MPS still utilize outdated, laborious methods for fabrication of these models. This lack of adoption is likely due to the initial barrier of access to the advanced technology of extrusion biofabrication. Many models above were fabricated on expensive, complex, custom-built platforms, which most labs do not have the expertise or funding to develop. These custom-built devices, while extremely powerful, limit the ability for reproducibility or collaboration. Likewise, initial commercially available extrusion biofabrication platforms were extremely expensive and difficult to use. This landscape is changing, however, with accessible, low-cost biofabrication platforms that incorporate the advanced technologies of these other platforms. The potential of these standardized, low-cost platforms is evident through advancements such as the thrombosis model depicted in Figure 4 and developed by Zhang et al. (22). With a standardized system like the one used in this work, advancements in the field can be easily reproduced and scaled. This capability will be especially important for the translation and mass production of these systems. A standardized platform also presents promise for translation of MPS into clinical practice for personalized medicine. Personalized therapies are becoming more and more relevant, and it will be necessary to develop methods to test compatibility of such therapies with individual patients (58,59). Consideration not only in MPS design but also manufacturing method, scalability and fabrication time must be considered. Standardized extrusion biofabrication platforms present great promise for this development and translation. In addition to standardization of these systems, continued improvement in extrusion biofabrication technology, as well as matrix bioinks, is necessary to provide enhanced resolution and manufacturing capabilities for the continued advancement of MPS.
Many of the current MPS fabricated with extrusion biofabrication incorporate some but not all advantages of this method. Systems focus on uniform patterning of 2D cells, or controlled architecture of vasculature channels, but do not always incorporate sensors for analysis, multiple cell types, 3D culture environments or design for application of additional mechanical or electrical stimuli. As more research institutions gain access to this technology and the field continues to mature, we predict the development of even more biomimetic, complex MPS that incorporate all of these features. Likewise, more multi-tissue platforms will likely be developed for the prediction of multiorgan toxicities and analysis. As these advancements rapidly develop, it would be useful to create a standardized method of analysis for success or failure of each of these systems.
These methods of analysis will need to be tailored for each tissue and tissue model developed, and should be based on functions measured in the clinic. MPS should be compared not only to current in vitro models, as many often are, but also to the in vivo systems or pathology the component is attempting to model. Clear markers of improvement and success should be determined prior to readout. Computational models should be developed to compare measurements in MPS to in vivo systems. Programs such as the National Institute of Health (NIH) MPS Program run by the NIH, the Defense Advanced Research Projects Agency (DARPA) and the U.S. Food and Drug Administration (FDA) not only provide funding for MPS research but also provide a set of standards and compounds for the testing of new models (60). This set of standards involves minimal functional requirements for each system and a set of training compounds based on known organ-specific toxicities in the human population (60). These types of standards should be further developed and applied to all projects regardless of funding for effective development of new systems (60).
In addition to standards for testing new systems, it is imperative to also consider the analysis of these systems and how data for this analysis will be collected. Common methods such as staining or anti-body based assays are often destructive and time-consuming (61). MPS must move toward real-time, non-destructive read-out capabilities for active monitoring and technologies with realistic scalability. Advances in soft sensor technologies and developments of bioinks for fabrication of integrated electrode arrays, such as the ones in Lewis et al., demonstrate an ideal approach to MPS design. More advancements in bioinks and incorporation of these soft sensor technologies are needed for the advancement of MPS.
Conclusions
Current methods of in vitro testing are highly ineffective and costly. MPS provide great promise to improve the effectiveness and decrease the cost of this testing. For truly advanced in vitro models, however, advanced fabrication methods must be implemented to recapitulate complex in vivo environments. Extrusion biofabrication platforms allow for the complexity, versatility and reproducibility necessary to fabricate truly physiologically relevant models. These systems have the potential to save billions of dollars and minimize exposure of harmful treatments to both animals and humans. Through automated fabrication via extrusion biofabrication, advancements in MPS have the potential to advance the rate of discovery of therapies to improve human health, hopefully creating a pace of advancement closer to Moore’s law.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/mps.2018.03.01). Margaret Prendergast, Ricardo Solorzano and Taciana Pereira work for Allevi, Inc. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Scannell JW, Blanckley A, Boldon H, et al. Diagnosing the decline in pharmaceutical R&D efficiency. Nat Rev Drug Discov 2012;11:191-200. [Crossref] [PubMed]
- Pharmaceutical Research and Manufacturers of America. 2016 biopharmaceutical research industry profile. Washington, DC: PhRMA, 2016.
- Fabre KM, Livingston C, Tagle DA. Organs-on-chips (microphysiological systems): tools to expedite efficacy and toxicity testing in human tissue. Exp Biol Med (Maywood) 2014;239:1073-7. [Crossref] [PubMed]
- Chandra P, Kengla C, Jin S. MIcrofabrication and 3D-Bioprinting of Organ-on-a-Chip. In: Murphy SV, Atala A, editor. Regenerative Medicine Technology: On-a-Chip Applications for Disease Modeling, Drug Discovery and Personalized Medicine. CRC Press: Taylor & Francis Group, 2016.
- Ravi M, Paramesh V, Kaviya SR, et al. 3D cell culture systems: advantages and applications. J Cell Physiol 2015;230:16-26. [Crossref] [PubMed]
- Benien P, Swami A. 3D tumor models: history, advances and future perspectives. Future Oncol 2014;10:1311-27. [Crossref] [PubMed]
- Malda J, Visser J, Melchels FP, et al. 25th anniversary article: Engineering hydrogels for biofabrication. Adv Mater 2013;25:5011-28. [Crossref] [PubMed]
- Prendergast ME, Solorzano RD, Cabrera D. Bioinks for biofabrication: current state and future perspectives. Journal of 3D Printing in Medicine 2016. [Epub ahead of print].
- Seol YJ, Kang HW, Lee SJ, et al. Bioprinting technology and its applications. Eur J Cardiothorac Surg 2014;46:342-8. [Crossref] [PubMed]
- Bhise NS, Manoharan V, Massa S, et al. A liver-on-a-chip platform with bioprinted hepatic spheroids. Biofabrication 2016;8:014101 [Crossref] [PubMed]
- Homan KA, Kolesky DB, Skylar-Scott MA, et al. Bioprinting of 3D Convoluted Renal Proximal Tubules on Perfusable Chips. Sci Rep 2016;6:34845. [Crossref] [PubMed]
- Yang Q, Lian Q, Xu F. Perspective: Fabrication of integrated organ-on-a-chip via bioprinting. Biomicrofluidics 2017;11:031301 [Crossref] [PubMed]
- Berthier E, Young EW, Beebe D. Engineers are from PDMS-land, Biologists are from Polystyrenia. Lab Chip 2012;12:1224-37. [Crossref] [PubMed]
- Esch EW, Bahinski A, Huh D. Organs-on-chips at the frontiers of drug discovery. Nat Rev Drug Discov 2015;14:248-60. [Crossref] [PubMed]
- Lind JU, Busbee TA, Valentine AD, et al. Instrumented cardiac microphysiological devices via multimaterial three-dimensional printing. Nat Mater 2017;16:303-8. [Crossref] [PubMed]
- Kim JA, Kim HN, Im SK, et al. Collagen-based brain microvasculature model in vitro using three-dimensional printed template. Biomicrofluidics 2015;9:024115 [Crossref] [PubMed]
- Zhang YS, Arneri A, Bersini S, et al. Bioprinting 3D microfibrous scaffolds for engineering endothelialized myocardium and heart-on-a-chip. Biomaterials 2016;110:45-59. [Crossref] [PubMed]
- Knowlton S, Joshi A, Yenilmez B, et al. Advancing cancer research using bioprinting for tumor-on-a-chip platforms. International Journal of Bioprinting 2016;2:3-8. [Crossref]
- Kizawa H, Nagao E, Shimamura M, et al. Scaffold-free 3D bio-printed human liver tissue stably maintains metabolic functions useful for drug discovery. Biochem Biophys Rep 2017;10:186-91. [Crossref] [PubMed]
- Lee H, Cho DW. One-step fabrication of an organ-on-a-chip with spatial heterogeneity using a 3D bioprinting technology. Lab Chip 2016;16:2618-25. [Crossref] [PubMed]
- Johnson BN, Lancaster KZ, Hogue IB, et al. 3D printed nervous system on a chip. Lab Chip 2016;16:1393-400. [Crossref] [PubMed]
- Zhang YS, Davoudi F, Walch P, et al. Bioprinted thrombosis-on-a-chip. Lab Chip 2016;16:4097-105. [Crossref] [PubMed]
- Horváth L, Umehara Y, Jud C, et al. Engineering an in vitro air-blood barrier by 3D bioprinting. Sci Rep 2015;5:7974. [Crossref] [PubMed]
- Kolesky DB, Homan KA, Skylar-Scott MA, et al. Three-dimensional bioprinting of thick vascularized tissues. Proc Natl Acad Sci U S A 2016;113:3179-84. [Crossref] [PubMed]
- Hinton TJ, Jallerat Q, Palchesko RN, et al. Three-dimensional printing of complex biological structures by freeform reversible embedding of suspended hydrogels. Sci Adv 2015;1:e1500758 [Crossref] [PubMed]
- Byun CK, Abi-Samra K, Cho YK, et al. Pumps for microfluidic cell culture. Electrophoresis 2014;35:245-57. [Crossref] [PubMed]
- Laser DJ, Santiago JG. A review of micropumps. Journal of Micromechanis and Microengineering 2004;14:R35. [Crossref]
- Materne EM, Maschmeyer I, Lorenz AK, et al. The multi-organ chip--a microfluidic platform for long-term multi-tissue coculture. J Vis Exp 2015;e52526 [PubMed]
- Park JY, Morgan M, Sachs AN, et al. Single cell trapping in larger microwells capable of supporting cell spreading and proliferation. Microfluid Nanofluidics 2010;8:263-8. [Crossref] [PubMed]
- Li Z, Mak SY, Sauret A, et al. Syringe-pump-induced fluctuation in all-aqueous microfluidic system implications for flow rate accuracy. Lab Chip 2014;14:744-9. [Crossref] [PubMed]
- Wang W, Gu C, Lynch KB, et al. High-pressure open-channel on-chip electroosmotic pump for nanoflow high performance liquid chromatography. Anal Chem 2014;86:1958-64. [Crossref] [PubMed]
- Wagner I, Materne EM, Brincker S, et al. A dynamic multi-organ-chip for long-term cultivation and substance testing proven by 3D human liver and skin tissue co-culture. Lab Chip 2013;13:3538-47. [Crossref] [PubMed]
- Truby RL, Lewis JA. Printing soft matter in three dimensions. Nature 2016;540:371-8. [Crossref] [PubMed]
- Xu Y, Wu X, Guo X, et al. The Boom in 3D-Printed Sensor Technology. Sensors (Basel) 2017;17:E1166 [Crossref] [PubMed]
- Balint R, Cassidy NJ, Cartmell SH. Conductive polymers: towards a smart biomaterial for tissue engineering. Acta Biomater 2014;10:2341-53. [Crossref] [PubMed]
- Kumar S, Ahlawat W, Kumar R, et al. Graphene, carbon nanotubes, zinc oxide and gold as elite nanomaterials for fabrication of biosensors for healthcare. Biosens Bioelectron 2015;70:498-503. [Crossref] [PubMed]
- Zhu K, Shin SR, van Kempen T, et al. Gold Nanocomposite Bioink for Printing 3D Cardiac Constructs. Advanced Functional Materials 2017;27:1605352 [Crossref]
- Gardin C, Piattelli A, Zavan B. Graphene in Regenerative Medicine: Focus on Stem Cells and Neuronal Differentiation. Trends Biotechnol 2016;34:435-7. [Crossref] [PubMed]
- Wei Zhu, Harris BT, Zhang LG. Gelatin methacrylamide hydrogel with graphene nanoplatelets for neural cell-laden 3D bioprinting. Conf Proc IEEE Eng Med Biol Soc 2016;2016:4185-8.
- Adams SD, Ashok A, Kanwar RK, et al. Integrated 3D printed scaffolds and electrical stimulation for enhancing primary human cardiomyocyte cultures. Bioprinting 2017;6:18-24. [Crossref]
- Svennersten K, Berggren M, Richter-Dahlfors A, et al. Mechanical stimulation of epithelial cells using polypyrrole microactuators. Lab Chip 2011;11:3287-93. [Crossref] [PubMed]
- Huh D, Hamilton GA, Ingber DE. From 3D cell culture to organs-on-chips. Trends Cell Biol 2011;21:745-54. [Crossref] [PubMed]
- Uzel SG, Pavesi A, Kamm RD. Microfabrication and microfluidics for muscle tissue models. Prog Biophys Mol Biol 2014;115:279-93. [Crossref] [PubMed]
- Huh DD. A human breathing lung-on-a-chip. Ann Am Thorac Soc 2015;12:S42-4. [Crossref] [PubMed]
- Davis CA, Zambrano S, Anumolu P, et al. Device-based in vitro techniques for mechanical stimulation of vascular cells: a review. J Biomech Eng 2015;137:040801 [Crossref] [PubMed]
- Kurokawa YK, George SC. Tissue engineering the cardiac microenvironment: Multicellular microphysiological systems for drug screening. Adv Drug Deliv Rev 2016;96:225-33. [Crossref] [PubMed]
- Xu D, Peltz G. Can Humanized Mice Predict Drug “Behavior” in Humans? Annu Rev Pharmacol Toxicol 2016;56:323-38. [Crossref] [PubMed]
- Soldatow VY, Lecluyse EL, Griffith LG, et al. In vitro models for liver toxicity testing. Toxicol Res (Camb) 2013;2:23-39. [Crossref] [PubMed]
- Skardal A, Shupe T, Atala A. Organoid-on-a-chip and body-on-a-chip systems for drug screening and disease modeling. Drug Discov Today 2016;21:1399-411. [Crossref] [PubMed]
- Choi K, Pfund WP, Andersen ME, et al. Development of 3D dynamic flow model of human liver and its application to prediction of metabolic clearance of 7-ethoxycoumarin. Tissue Eng Part C Methods 2014;20:641-51. [Crossref] [PubMed]
- Snyder JE, Hamid Q, Wang C, et al. Bioprinting cell-laden matrigel for radioprotection study of liver by pro-drug conversion in a dual-tissue microfluidic chip. Biofabrication 2011;3:034112 [Crossref] [PubMed]
- Faulkner-Jones A, Fyfe C, Cornelissen DJ, et al. Bioprinting of human pluripotent stem cells and their directed differentiation into hepatocyte-like cells for the generation of mini-livers in 3D. Biofabrication 2015;7:044102 [Crossref] [PubMed]
- Ma X, Qu X, Zhu W, et al. Deterministically patterned biomimetic human iPSC-derived hepatic model via rapid 3D bioprinting. Proc Natl Acad Sci U S A 2016;113:2206-11. [Crossref] [PubMed]
- Skardal A, Murphy SV, Devarasetty M, et al. Multi-tissue interactions in an integrated three-tissue organ-on-a-chip platform. Sci Rep 2017;7:8837. [Crossref] [PubMed]
- Xiao S, Coppeta JR, Rogers HB, et al. A microfluidic culture model of the human reproductive tract and 28-day menstrual cycle. Nat Commun 2017;8:14584. [Crossref] [PubMed]
- Yi HG, Lee H, Cho DW. 3D Printing of Organs-On-Chips. Bioengineering (Basel) 2017;4:E10 [Crossref] [PubMed]
- Lee SH, Sung JH. Organ-on-a-Chip Technology for Reproducing Multiorgan Physiology. Adv Healthc Mater 2018;7. [PubMed]
- Hirsch T, Rothoeft T, Teig N, et al. Regeneration of the entire human epidermis using transgenic stem cells. Nature 2017;551:327-32. [Crossref] [PubMed]
- National Cancer Institute. CAR T-Cell Therapy Approved for Some Children and Young Adults with Leukemia. Cancer Currents Blog 2017. Available online: https://www.cancer.gov/news-events/cancer-currents-blog/2017/tisagenlecleucel-fda-childhood-leukemia
- Sutherland ML, Fabre KM, Tagle DA. The National Institutes of Health Microphysiological Systems Program focuses on a critical challenge in the drug discovery pipeline. Stem Cell Res Ther 2013;4:I1. [Crossref] [PubMed]
- Capulli AK, Tian K, Mehandru N, et al. Approaching the in vitro clinical trial: engineering organs on chips. Lab Chip 2014;14:3181-6. [Crossref] [PubMed]
Cite this article as: Prendergast ME, Montoya G, Pereira T, Lewicki J, Solorzano R, Atala A. Microphysiological Systems: automated fabrication via extrusion bioprinting. Microphysiol Syst 2018;2:2.


