
SARS-CoV-2-related vascular injury: mechanisms, imaging and models
Introduction
The outbreak of the novel coronavirus disease 2019 (COVID-19) has since reached pandemic status, with over 93 million cumulative confirmed cases as of January 17th 2021 (1). Vascular complications, including thrombotic microangiopathy, vasculopathy, and intravascular coagulopathy, are of growing concern in patients with COVID-19 as they can lead to devastating vital organ injury at various stages of the disease course (2). Despite the growing concern, there is still limited research on the downstream effects of endothelial cell infection with severe acute respiratory coronavirus 2 (SARS-CoV-2). Considering the potential for virally-driven vascular injury, there is a utility in studying the characteristics that influence the pro-inflammatory hypercoagulability seen among some proportion of patients with COVID-19. Investigating the consequences and long-term disease processes of COVID-19 patients with damage to vasculature will be crucial for informing future avenues of clinical care.
In this concise editorial, we provide an overall account of the cellular mechanisms currently thought to contribute to vascular pathology in patients with COVID-19. We also discuss the various vascular diseases involved in the COVID-19 clinical course. Finally, we explore the use of emerging imaging technologies in conjunction with models of tissue injury to better visualize and understand SARS-CoV-2-driven vascular damage.
Mechanism of SARS-CoV-2 vascular injury
The pathogenesis of SARS-CoV-2 offers insight into how the virus directly interacts with host cells and the immune system, providing greater context for subsequent vascular tissue damage.
Viral infection with SARS-CoV-2 is initiated when the S spike, a surface glycoprotein on SARS-CoV-2, binds human angiotensin-converting enzyme 2 (ACE2), which is highly expressed in the tissue of the lung, heart, and kidney (3). Within the renin-angiotensin signaling (RAS) pathway, ACE2 facilitates the conversion of angiotensin II (ANGII) into angiotensin 1–7. When bound by the S spike, the transmembrane ACE2 receptor signaling cascade is functionally impaired as the virus competes with native mitogens involved in the RAS pathway. Figure 1 provides context for how this disruption in angiotensin metabolism leads to aberrantly high levels of ANGII. High levels of ANGII ultimately favor pro-inflammatory and pro-fibrotic chemotactic signaling, which leads to greater local vascular tissue remodeling in patients with COVID-19.
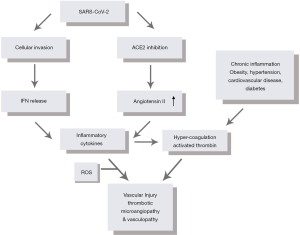
After SARS-CoV-2 gains entry into ACE2-expressing host cells, the negative effects on vascular tissue are amplified. In response to viral invasion, human cells typically respond by synthesizing and secreting interferons (IFNs). The release of IFNs alerts neighboring cells to the presence of a harmful pathogen and also activates a signaling cascade that shifts cells into an “antiviral state” (4,5). Research regarding the effects of coronaviruses on the IFN signal transduction pathway, however, suggests that various components of this pathway are downregulated after inoculation. Reduced IFN activity preserves the host’s intracellular activity, allowing SARS-CoV-2 to exploit unusually energetic conditions to drive virion replication and exocytosis (6,7). SARS-CoV-2’s interference in IFN signaling delays viral recognition and can lead to a higher viral load, resulting in an innate immune response that releases large quantities of pro-inflammatory cytokines (8,9). Subsequent induction of reactive oxygen species (ROS) functioning downstream to aberrant cytokine signaling further compromises the endothelial cell structure. The combination of over-expressed inflammatory cytokines and ROS result in a “cytokine storm,” shown in Figure 1. The “cytokine storm” leads to the recruitment of additional immune cells including macrophages, neutrophils, natural killer (NK) cells, and dendritic cells, releasing activation factors within the vasculature and damaging endothelial tissue in the process (5).
After extended periods of replication within host epithelial cells, SARS-CoV-2 may also affect the adaptive immune response, consequently prolonging infection. In patients with severe COVID-19, key components of the adaptive immune response, including CD4+ cells, CD8+ cells, B cells, NK cells, monocytes, eosinophils, and basophils, showed a marked decrease in circulation (9-13). There is additional evidence to suggest a virally driven imbalance in naïve and memory T cells following SARS-CoV-2 infection, which may contribute to the hyper inflammation and prolonged clinical course seen in COVID-19 patients (9,14,15).
Thus, the mechanism of SARS-CoV-2 infection reveals the profound impact it has on the human immune system. The additive effects of SARS-CoV-2’s binding, entry, and proliferation within host cells collectively interfere with the human immune responses and weaken vascular environments, laying a foundation upon which more severe damages can be elicited.
Vascular injury in patients with COVID-19
In a vascular environment weakened by SARS-CoV-2 infection, a patient’s vascular clinical course is largely dependent on the degree of virally-driven inflammation and coagulation as well as the damage dealt by pre-existing conditions.
Inflammation, coagulation abnormalities and endothelial injury
Preliminary research of patients with COVID-19 suggests that SARS-CoV-2 infection is associated with an intense inflammatory response. This response is mediated by the release of pro-inflammatory cytokines, including tumor necrosis factor-α (TNF-α) and interleukins 1 and 6 (IL-1, IL-6) (8). These cytokines are implicated in the formation and activation of mononuclear cells, known to initiate coagulation through the release of thrombin (16). Accordingly, distinctive vascular features are observed in the pathology of peripheral lung in patients with COVID-19, including evidence of thrombotic microangiopathy, vasculopathy, and intravascular coagulopathy (16-19). When compared to patients with influenza, patients hospitalized with COVID-19 had nine times greater prevalence of alveolar-capillary microthrombi on histologic tissue samples (19). Ultimately, these data suggest that infection with SARS-CoV-2 results in hyper inflammation and hypercoagulation.
As blood viscosity increases in association with hyper inflammation, the risk of thrombus formation rises significantly. Blood vessels are especially susceptible to damage in areas of plaque formation as hemodynamic fluctuations can induce downstream thrombosis or ischemia (20). In fact, several studies have reported elevated levels of D-dimer, a common and nonspecific biomarker of ongoing inflammation and thrombus formation, in patients with COVID-19 (15,16). Elevated levels of the biomarker C-reactive protein (CRP), an acute phase reactant associated with ongoing inflammation within the body, was also witnessed in patients with COVID-19 (21). There is additional evidence suggesting that patients with COVID-19 accumulate vascular damage within a pro-inflammatory and hypercoagulable state, corresponding to documented evidence of elevated acute phase reactant and prolonged prothrombin presence. For example, among 96 detailed reports of patients with COVID-19 who suffered an acute stroke, CRP, D-dimer, and ferritin were found to be significantly elevated (22). Similarly, in a study of SARS-CoV-2 and stroke in the New York health care system, the median of D-dimer was >10,000 ng/mL in stroke patients with positive COVID-19 as compared to stroke patients without COVID-19, whose median value of D-dimer was only 526 ng/mL (23). Thus, through the overwhelming amount of evidence presented above, we understand that infection with SARS-CoV-2 triggers hyper inflammation and hypercoagulation, further deteriorating the vascular health of patients with COVID-19.
Comorbidities and SARS-CoV-2 vascular injury
In a state of virally-induced hyper inflammation and hypercoagulation, individuals with comorbidities such as cardiovascular disease, diabetes, hypertension, and obesity are at higher risk of a poor clinical course following SARS-CoV-2 infection (24-26). Prior to infection, patients with a history of these comorbidities are likely in a chronic state of low-grade inflammation, thereby compromising endothelial function over time, resulting in arterial wall stiffening (24). Upon infection, existing endothelial dysfunction facilitates the infection of cells with SARS-CoV-2 while chronic inflammation simultaneously contributes to hypercoagulability. These two factors act together, increasing the odds of a severe clinical course complicated by microangiopathic injury, which is highlighted in Figure 1.
Weakened vascular health brought about by pre-existing conditions in addition to the hyperinflammatory, hypercoagulable state resulting from SARS-CoV-2 infection may promote underlying damage to the vasculature and surrounding tissue in multiple vital organs (26). Elevated markers of cardiac injury, including troponin I and creatine kinase (CK), have been reported in patients endorsing chest pain with COVID-19 (25). Additional cardiovascular complications associated with COVID-19 include arrhythmias, fulminant myocarditis, and heart failure (21,25). Cerebrovascular disease, including transient ischemic attack (TIA) and stroke, may also complicate the clinical course of patients with COVID-19 (22,27-29). Importantly, ischemic strokes of the vertebrobasilar territory have been recorded in unexpectedly high frequencies among a cohort of 1,683 patients averaging 5 days between initial presentation with COVID-19 symptoms and stroke onset (29). In addition to strokes, thrombotic events and subsequent tissue injury are seen throughout the body as a result of infection, leading to worse outcomes (17). Consequences of hypercoagulability of COVID-19 occur in the arterial as well as the venous system, as deep vein thrombosis (DVT) and pulmonary embolism (PE) have been well recognized as complications in cases of severe COVID-19 (28).
Taken together, it becomes clear that SARS-CoV-2 is heavily involved in generating a hyper inflammatory and hypercoagulable state. This state can very quickly lead to the formation of thrombi, which threatens regions of the vasculature weakened by chronic inflammation. As infection with SARS-CoV-2 has such deadly implications, it is important that we identify and implement technologies that monitor fluctuations in vascular health.
Emerging vasculature imaging for COVID-19 complications
When hypercoagulability and/or thrombotic microangiopathic injury is suspected, the diagnostic choice of imaging modality is based on a combination of clinical presentation and patient-specific factors, for example, allergies to contrast and ability to tolerate exam. For COVID-19 patients with suspected vessel occlusions in the extremities (DVT) and abdomen (renal vasculature), we assert that doppler ultrasound (DUS) is the gold standard for diagnostic imaging.
DUS relies on the detection of ultrasound signals to probe the tissue’s anatomy and functions. Leveraging the Doppler effect, DUS technology is capable of reporting hematocrit values in addition to the quality of blood flow in stenotic or occluded vasculature (29). By monitoring vessel patency and red blood cell concentration, DUS lends medical professionals the ability to locate the point of thrombotic vessel occlusion (Figure 2A) responsible for causing either ischemia or interstitial congestion in coagulopathic patients (32,33). After a patient has recovered, DUS can be used to detect endoleak following endovascular repair in microvessels (34). Thus, in patients recovering from COVID-19, DUS might be a useful tool in detecting long-term vascular remodeling. Additionally, the non-invasive and relatively inexpensive aspects of DUS permit greater clinical access and more rapid implementation.
While DUS has already shown great utility in clinical application, we believe that further development of novel biomedical imaging technologies might allow medical professionals to glean greater details from images captured in the clinic. Photoacoustic tomography (PAT), for example, is an emerging technology that has shown promising utility in several biomedical applications (35,36). PAT relies on light-induced ultrasound emission through transient thermoelastic expansion (37). When paired with ultrasound technology, PAT can detect the location and age of DVT (Figure 2B) as well as report thrombosis composition following microbubble-assisted sonothrombolysis treatment (38,39). These chemical and functional details can greatly impact a physician’s treatment plan when caring for a COVID-19 patient with complications related to thrombotic microangiopathy. Advanced age of a DVT, for example, increases the likelihood of the formation of an embolism, which can alert clinicians to imminent pulmonary ischemia threatening the lung tissue. Thrombosis composition might also influence a physician’s choice in dosage and timing of anticoagulant treatment. Although PAT is not yet extensively used in clinical practice, the unique qualities of this novel imaging technology may provide detailed information on vascular injury and response to treatment, becoming an important tool in ongoing COVID-19 patient care and research.
Potential in vitro tissue models for vascular injury in COVID-19 patients
While there are great prospects for the use of the aforementioned imaging modalities in the clinical diagnosis of COVID-19-related vascular complications, research models of endothelial injury resulting from SARS-CoV-2 infection will also be essential in understanding the mechanisms contributing to devastating complications. While small animal models such as rodents have been popular for imaging studies in the past, COVID-19 presents a unique challenge: no existing animal model perfectly mimicks the human immune response to SARS-CoV-2 infection. To overcome this challenge, there is utility in studying transgenic animal models as well as human-based tissue models to conduct imaging research on vascular injury with COVID-19. One such model is already under development for COVID-19 vaccination testing (40). By breeding and infecting transgenic ACE2 mice with SARS-CoV-2, researchers were able to induce human symptoms in mice. Imaging these transgenic models might be extremely informative, allowing the aforementioned imaging modalities to become a routine part of COVID-19 symptom monitoring. However, it should be noted that these transgenic models still do not perfectly model human immune responses to COVID-19, which may lead to false conclusions surrounding imaging diagnostic data. Thus, we suggest looking to alternative solutions, namely innovative models that are able to exactly model human physiology without compromising the health of patients and risking the spread of COVID-19.
An alternative to animal models is to use engineered human tissue. One exciting platform is the microfluidic organ-on-a-chip system (41,42), which has recently been combined with three-dimensional (3D) bioprinting to produce high-content in vitro human-based models (43). The organ-on-a-chip platform enables the modeling of vascular structures using microfluidics and microfabrication techniques. Microfluidic devices provide dynamic flows to relevant cells, such as endothelial cells, facilitating the real-time imaging of vascular responses to mechanical and/or chemical influences (44,45). In the lung-on-a-chip model, based on the alveolar and capillary interface, endothelial cells are cultured on the bottom of a porous membrane-based device while the alveolar epithelial cells are seeded above the same membrane (Figure 3A) (46). To simulate cyclic breathing, both cell layers undergo repetitive simultaneous stretching. Reproducible organ-level responses to bacterial infection and inflammation were achieved in this device. However, the most current organ-on-a-chip systems are compartmentalized and locally planar, limiting their ability to model certain complex aspects of the tissues and organs they model.
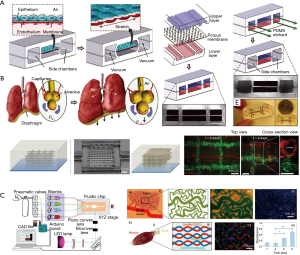
To resolve this issue, 3D bioprinting technology has emerged as a pivotal strategy to build volumetric constructs in precise geometries with various cell types and extracellular matrix (ECM) materials in an automated manner (49,50). In vascular 3D bioprinting, bioinks containing vascular cells and biochemical factors can be used to produce volumetric tissue constructs containing perfusable vascular networks. Extrusion bioprinting is one of the most commonly used methods for vascular bioprinting and is based on thermo-, ionic, or photo-crosslinking (51). To fabricate the heterogeneous vasculature with gelatin methacryloyl (GelMA) as a matrix, Pluronic F127 was used as the sacrificial bioink and was subsequently selectively removed to achieve the perfusable embedded microchannels seen in Figure 3B (47). Vat photopolymerization is a high-speed and high-resolution bioprinting method based on photocrosslinking of hydrogel bioinks layer-by-layer when exposure to sequentially digitally patterned light (50). For example, Miller and colleagues recently reported the bioprinting of the multi-vascular network at the organ-scale using such a technique (52). A microfluidic platform to fabricate multi-material constructs containing microvasculature in different tissue types has also been developed (Figure 3C) (48).
Of note, 3D bioprinting is not entirely separate from microfabricated organ-on-a-chip platforms, and they can be easily integrated to enable the dynamic environments supplied by the latter and the volumetric complexity provided by the former (43,53). As these strategies achieve a model of desired human tissues on a relatively small scale, researchers gain access to a more accurate and higher-throughput alternative to animal modeling. More importantly, the engineered tissue chip technology can be used in conjunction with the aforementioned imaging modalities as it reduces the necessity for high penetration depth, including traditional optical microcopy technologies. With aid from novel imaging modalities and modeling technologies, a human-based vascular tissue model that is immunocompetent (54-57), can likely be developed and optimized. In fact, one research group has already identified Amodiaquine as an especially potent antagonist of SARS-CoV-2’s entry into host cells by repurposing a pulmonary organ-on-a-chip model previously used for influenza drug optimization (58). As a result, through the use of organ-on-a-chip modeling in conjunction with 3D bioprinting, we can achieve a better understanding of how SARS-COV-2 infects and damages human vasculature without sacrificing model accuracy and participant safety.
Conclusions
In summary, there is accumulating evidence suggesting that thrombosis and vascular injury are connected with SARS-CoV-2 infection. SARS-CoV-2’s binding affinity for ACE2, its ability to evade innate immunity upon entry, and its role in disrupting the adaptive immune response collectively act together to compromise the human vascular system. While the exact mechanism of thrombotic microangiopathic vascular injury in patients with COVID-19 remains to be explored, available evidence suggests that virally-driven hyperinflammation and hypercoagulation as well as comorbidities associated with chronic inflammation and endothelial injury put patients with COVID-19 at greater risk of poor clinical outcome. In an effort to further the understanding of COVID-19 and its impact on vascular health, vasculature imaging presents itself as an important tool to better visualize vascular damage, aid in early diagnosis, guide treatment, and monitor the disease progression of patients with COVID-19. When used in parallel, DUS and PAT have great advantages in assessing vascular injury and advising therapy. Additionally, 3D bioprinting and organ-on-a-chip are two emerging technologies capable of modeling vascular tissue injury in COVID-19 and assessing the viability of DUS and PAT in diagnosis. As the global community continues to work to contain the spread of COVID-19, much is still unknown about this virus and its impact on the vascular system. The effects of this pandemic on the human population will certainly not end with the distribution of a vaccine, so we must continue to research COVID-19 and its potential to cause long-term vascular harm to those who have recovered from it.
Acknowledgments
Funding: This work was supported in part by National Institute of Health (R01 EB028143, R01 NS111039, RFA NS115581, R01 GM134036, R21 EB027304, R21 EB027981, R43 CA243822, R43 CA239830, R44 HL138185); Duke Institute of Brain Science Incubator Award; Chan Zuckerberg Initiative Deep Tissue Imaging Grant; and American Heart Association Collaborative Sciences Award (18CSA34080277).
Footnote
Provenance and Peer Review: This article was a free submission to the journal. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/mps-20-6). YSZ serves as the unpaid Editor-in-Chief of Microphysiological Systems. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- World Health Organization. Coronavirus disease (COVID-19): weekly epidemiological update. January 2021.
- Bansal R, Gubbi S, Muniyappa R. Metabolic syndrome and COVID 19: endocrine-immune-vascular interactions shapes clinical course. Endocrinology 2020;161:bqaa112.
- Varga Z, Flammer AJ, Steiger P, et al. Endothelial cell infection and endotheliitis in COVID-19. Lancet 2020;395:1417-8. [Crossref] [PubMed]
- Borden EC, Sen GC, Uze G, et al. Interferons at age 50: past, current and future impact on biomedicine. Nat Rev Drug Discov 2007;6:975-90. [Crossref] [PubMed]
- Kindler E, Thiel V. SARS-CoV and IFN: too little, too late. Cell Host Microbe 2016;19:139-41. [Crossref] [PubMed]
- Channappanavar R, Fehr AR, Vijay R, et al. Dysregulated type I interferon and inflammatory monocyte-macrophage responses cause lethal pneumonia in SARS-CoV-infected mice. Cell Host Microbe 2016;19:181-93. [Crossref] [PubMed]
- Weiss SR. Forty years with coronaviruses. J Exp Med 2020;217:e20200537. [Crossref] [PubMed]
- Mehta P, McAuley DF, Brown M, et al. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet 2020;395:1033-4. [Crossref] [PubMed]
- Qin C, Zhou L, Hu Z, et al. Dysregulation of immune response in patients with coronavirus 2019 (COVID-19) in Wuhan, China. Clin Infect Dis 2020;71:762-8. [Crossref] [PubMed]
- Tan M, Liu Y, Zhou R, et al. Immunopathological characteristics of coronavirus disease 2019 cases in Guangzhou, China. Immunology 2020;160:261-8. [Crossref] [PubMed]
- Xu Z, Shi L, Wang Y, et al. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med 2020;8:420-2. [Crossref] [PubMed]
- Zhang B, Zhou X, Zhu C, et al. Immune phenotyping based on the neutrophil-to-lymphocyte ratio and IgG level predicts disease severity and outcome for patients with COVID-19. Front Mol Biosci 2020;7:157. [Crossref] [PubMed]
- Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020;395:497-506. [Crossref] [PubMed]
- Catanzaro M, Fagiani F, Racchi M, et al. Immune response in COVID-19: addressing a pharmacological challenge by targeting pathways triggered by SARS-CoV-2. Signal Transduct Target Ther 2020;5:84. [Crossref] [PubMed]
- Perlman S, Dandekar AA. Immunopathogenesis of coronavirus infections: implications for SARS. Nat Rev Immunol 2005;5:917-27. [Crossref] [PubMed]
- Levi M, Thachil J, Iba T, et al. Coagulation abnormalities and thrombosis in patients with COVID-19. Lancet Haematol 2020;7:e438-40. [Crossref] [PubMed]
- Henry BM, Vikse J, Benoit S, et al. Hyperinflammation and derangement of renin-angiotensin-aldosterone system in COVID-19: A novel hypothesis for clinically suspected hypercoagulopathy and microvascular immunothrombosis. Clin Chim Acta 2020;507:167-73. [Crossref] [PubMed]
- Liao D, Zhou F, Luo L, et al. Haematological characteristics and risk factors in the classification and prognosis evaluation of COVID-19: a retrospective cohort study. Lancet Haematol 2020;7:e671-8. [Crossref] [PubMed]
- Ackermann M, Verleden SE, Kuehnel M, et al. Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in Covid-19. N Engl J Med 2020;383:120-8. [Crossref] [PubMed]
- Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet 2020;395:1054-62. [Crossref] [PubMed]
- Aggarwal S, Garcia-Telles N, Aggarwal G, et al. Clinical features, laboratory characteristics, and outcomes of patients hospitalized with coronavirus disease 2019 (COVID-19): early report from the United States. Diagnosis (Berl) 2020;7:91-6. [Crossref] [PubMed]
- Ellul MA, Benjamin L, Singh B, et al. Neurological associations of COVID-19. Lancet Neurol 2020;19:767-83. [Crossref] [PubMed]
- Yaghi S, Ishida K, Torres J, et al. SARS-CoV-2 and stroke in a New York Healthcare System. Stroke 2020;51:2002-11. [Crossref] [PubMed]
- Caci G, Albini A, Malerba M, et al. COVID-19 and obesity: dangerous liaisons. J Clin Med 2020;9:2511. [Crossref] [PubMed]
- Guzik TJ, Mohiddin SA, Dimarco A, et al. COVID-19 and the cardiovascular system: implications for risk assessment, diagnosis, and treatment options. Cardiovasc Res 2020;116:1666-87. [Crossref] [PubMed]
- Gupta A, Madhavan MV, Sehgal K, et al. Extrapulmonary manifestations of COVID-19. Nat Med 2020;26:1017-32. [Crossref] [PubMed]
- Fan H, Tang X, Song Y, et al. Influence of COVID-19 on cerebrovascular disease and its possible mechanism. Neuropsychiatr Dis Treat 2020;16:1359-67. [Crossref] [PubMed]
- McFadyen JD, Stevens H, Peter K. The emerging threat of (micro)thrombosis in COVID-19 and its therapeutic implications. Circ Res 2020;127:571-87. [Crossref] [PubMed]
- Pialot B, Gachelin J, Tanter M, et al. Flow rate and low hematocrit measurements for in vitro blood processing with doppler ultrasound. IEEE Trans Ultrason Ferroelectr Freq Control 2020;67:1293-302. [Crossref] [PubMed]
- Zhang X, Macoskey JJ, Ives K, et al. Non-invasive thrombolysis using microtripsy in a porcine deep vein thrombosis model. Ultrasound Med Biol 2017;43:1378-90. [Crossref] [PubMed]
- Sivasubramanian K, Das D, Pramanik M. High frame rate photoacoustic imaging of blood clots. In: Photons Plus Ultrasound: Imaging and Sensing 2019. International Society for Optics and Photonics, 2019;10878:108785Y.
- Nakamura H, Inoue Y, Kudo T, et al. Detection of venous emboli using Doppler ultrasound. Eur J Vasc Endovasc Surg 2008;35:96-101. [Crossref] [PubMed]
- Valdueza JM, Schultz M, Harms L, et al. Venous transcranial Doppler ultrasound monitoring in acute dural sinus thrombosis. Report of two cases. Stroke 1995;26:1196-9. [Crossref] [PubMed]
- Tomczak J, Gabriel M, Snoch-Ziółkiewicz M, et al. Angio PLanewave UltraSensitive Imaging (Angio PL.U.S.) as an innovative Doppler ultrasound technique with a potential to follow up endoleaks after endovascular aneurysm repair (EVAR). Ultrasound Med Biol 2020;46:1707-14. [Crossref] [PubMed]
- Wang S, Lin J, Wang T, et al. Recent Advances in Photoacoustic Imaging for Deep-Tissue Biomedical Applications. Theranostics 2016;6:2394-413. [Crossref] [PubMed]
- Lediju Bell MA. Photoacoustic imaging for surgical guidance: Principles, applications, and outlook. J Appl Phys 2020;128:060904. [Crossref] [PubMed]
- Beard P. Biomedical photoacoustic imaging. Interface Focus 2011;1:602-31. [Crossref] [PubMed]
- Das D, Pramanik M. Combined ultrasound and photoacoustic imaging of blood clot during microbubble-assisted sonothrombolysis. J Biomed Opt 2019;24:1-8. [Crossref] [PubMed]
- Karpiouk AB, Aglyamov SR, Mallidi S, et al. Combined ultrasound and photoacoustic imaging to detect and stage deep vein thrombosis: phantom and ex vivo studies. J Biomed Opt 2008;13:054061. [Crossref] [PubMed]
- Gu H, Chen Q, Yang G, et al. Adaptation of SARS-CoV-2 in BALB/c mice for testing vaccine efficacy. Science 2020;369:1603-7. [PubMed]
- Bhatia SN, Ingber DE. Microfluidic organs-on-chips. Nat Biotechnol 2014;32:760-72. [Crossref] [PubMed]
- Zhang YS. The ultimate in personalized medicine: your body on a chip. IEEE Spectrum 2019. Available online: (accessed Sep. 13, 2020).https://spectrum.ieee.org/biomedical/diagnostics/the-ultimate-in-personalized-medicine-your-body-on-a-chip
- Zhang YS, Khademhosseini A. Engineering in vitro human tissue models through bio-design and manufacturing. Bio-Des Manuf 2020;3:155-9. [Crossref]
- Ribas J, Sadeghi H, Manbachi A, et al. Cardiovascular organ-on-a-chip platforms for drug discovery and development. Appl In Vitro Toxicol 2016;2:82-96. [Crossref] [PubMed]
- Zhang YS, Davoudi F, Walch P, et al. Bioprinted thrombosis-on-a-chip. Lab Chip 2016;16:4097-105. [Crossref] [PubMed]
- Huh D, Matthews BD, Mammoto A, et al. Reconstituting organ-level lung functions on a chip. Science 2010;328:1662-8. [Crossref] [PubMed]
- Kolesky DB, Truby RL, Gladman AS, et al. 3D bioprinting of vascularized, heterogeneous cell-laden tissue constructs. Adv Mater 2014;26:3124-30. [Crossref] [PubMed]
- Miri AK, Nieto D, Iglesias L, et al. Microfluidics-enabled multimaterial maskless stereolithographic bioprinting. Adv Mater 2018;30:e1800242. [Crossref] [PubMed]
- Heinrich MA, Liu W, Jimenez A, et al. 3D bioprinting: from benches to translational applications. Small 2019;15:e1805510. [Crossref] [PubMed]
- Li W, Mille LS, Robledo JA, et al. Recent advances in formulating and processing biomaterial inks for vat polymerization-based 3D printing. Adv Healthc Mater 2020;9:e2000156. [Crossref] [PubMed]
- Kolesky DB, Homan KA, Skylar-Scott MA, et al. Three-dimensional bioprinting of thick vascularized tissues. Proc Natl Acad Sci U S A 2016;113:3179-84. [Crossref] [PubMed]
- Skylar-Scott MA, Uzel SGM, Nam LL, et al. Biomanufacturing of organ-specific tissues with high cellular density and embedded vascular channels. Sci Adv 2019;5:eaaw2459. [Crossref] [PubMed]
- Parrish J, Lim K, Zhang B, et al. New frontiers for biofabrication and bioreactor design in microphysiological system development. Trends Biotechnol 2019;37:1327-43. [Crossref] [PubMed]
- Maharjan S, Cecen B, Zhang YS. 3D Immunocompetent Organ-on-a-Chip Models. Small Methods 2020;4:2000235. [Crossref] [PubMed]
- Massa S, Sakr MA, Seo J, et al. Bioprinted 3D vascularized tissue model for drug toxicity analysis. Biomicrofluidics 2017;11:044109. [Crossref] [PubMed]
- Sharifi F, Htwe SS, Righi M, et al. A foreign body response-on-a-chip platform. Adv Healthc Mater 2019;8:e1801425. [Crossref] [PubMed]
- Zhang YS, Oklu R, Albadawi H. Bioengineered in vitro models of thrombosis: methods and techniques. Cardiovasc Diagn Ther 2017;7:S329-35. [Crossref] [PubMed]
- Si L, Bai H, Rodas M, et al. Human organ chip-enabled pipeline to rapidly repurpose therapeutics during viral pandemics. bioRxiv 2020. doi: 10.1101/2020.04.13.039917. [Crossref]
Cite this article as: Humayun L, Smith C, Li W, Zhang YS, Park C, Feng W, Yao J. SARS-CoV-2-related vascular injury: mechanisms, imaging and models. Microphysiol Syst 2021;5:1.



